Cardiovascular diseases are the leading causes of death and disability worldwide. We study the role of the immune system in heart disease with a focus on innate immune cells and lymphatics. We also use a biomaterials approach to develop new ways of delivering therapies to the heart. All our work is ultimately focused on patients, specifically areas of cardiac pathophysiology, that are understudied and methods to target therapies to the heart better.

Rebecca Levit, MD (PI)
Projects
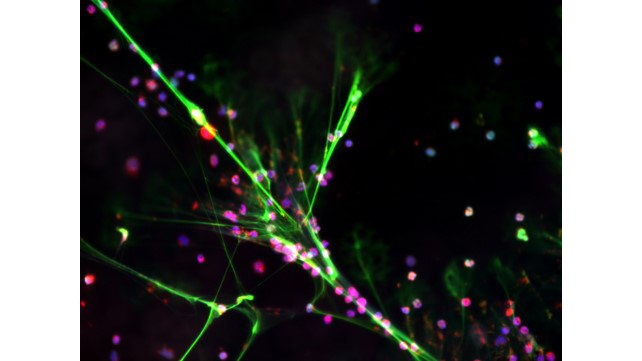
Neutrophils in cardiac injury
Neutrophils are the most common immune cell in humans and they rapidly infiltrate the heart after injury. We study many physiologic functions of neutrophils that can be detrimental when directed to the heart. The picture shows neutrophils undergoing NETosis, a process where they extrude their DNA into the extracellular space. This causes more damage to the heart and blood vessels and amplifies inflammation. We utilize transcriptomics, animal models, and human-derived cells to better understand neutrophils in cardiovascular biology.
Project funding: NIH-NHLBI R01HL140223
Key publications:
Sayegh MN, et al. Hydrogel delivery of purinergic enzymes improves cardiac ischemia/reperfusion injury. Journal of Molecular and Cellular Cardiology. 176. (2023); 98-109.
Sayegh MN, Cooney KA, Han WM, Wang L, Strobel F, Hansen LA, Garcia AJ, Levit RD. Hydrogel Strategy to Augment Tissue Adenosine to Improve Hindlimb Perfusion. Arterioscler Thromb Vasc Biol. 2021.
Xu, K., et al. (2019). "Adenosine from a biologic source regulates neutrophil extracellular traps (NETs)." Journal of Leukocyte Biology.
Shin EY, Wang L, Zemskova M, Deppen J, Xu K, Garcia AJ, Tirouvanziam R, Levit RD. Adenosine production by biomaterial-supported mesenchymal stromal cells reduces the innate inflammatory response in cardiac ischemia reperfusion. JAHA, 2018.
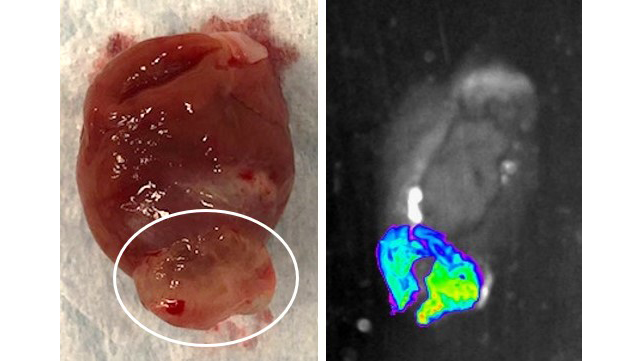
Hydrogel directed cardiac therapeutics
Some cardiac therapies are toxic to other organs and do not work if they are distributed systemically. Amiodarone, a powerful drug to treat arrythmias, is very effective in the heart but toxic to the lungs, liver and thyroid. To get therapeutic drug levels in the heart, other organs are at risk. Gene therapies use viral vectors to deliver gene-altering therapies to the heart but have not worked in clinical trial because of inefficient cardiac delivery. We are working to develop hydrogels to better focus and concentrate therapies to the heart. We are also working on a hydrogel delivery device to allow minimally invasive delivery of these therapies to patients.
Project funding: NIH-NHLBI R43 HL149495
Key publications:
Garcia JR, Campbell PF, Kumar G, Langberg JJ, Cesar L, Deppen JN, Shin EY, Bhatia NK, Wang L, Xu K, Schneider F, Robinson B, García AJ, Levit RD. Minimally Invasive Delivery of Hydrogel-Encapsulated Amiodarone to the Epicardium Reduces Atrial Fibrillation. Circulation: Arrhythmia and Electrophysiology. 2018;11.
Garcia JR, Campbell PF, Kumar G, Langberg JJ, Cesar L, Wang L, Garcia AJ, Levit RD. A minimally invasive, translational method to deliver hydrogels to the heart through the pericardial space. JACC:BTS 2017.

Cardiac lymphatics
The heart has a lymphatic vascular system distinct from blood vasculature. Cardiac lymphatics play a key role in regulation of immunity and interstitial fluids. In heart transplantation, the lymphatic vessels are severed and not re-connected which may contribute to rejection and loss of heart function. We are utilizing patient samples and animal models to both understand the role of lymphatics in heart transplants and develop new therapies that target cardiac lymphatics.
Project funding: Enduring Hearts Foundation and the American Heart Association
Key publications: Ginn SC, Dragan D, Niclou BA, Wang L, Ko YA, vanBeuningen A, Burke MA, Levit RD. Cardiac lymphatics predict survival after heart transplantation. JAHA. 2026.
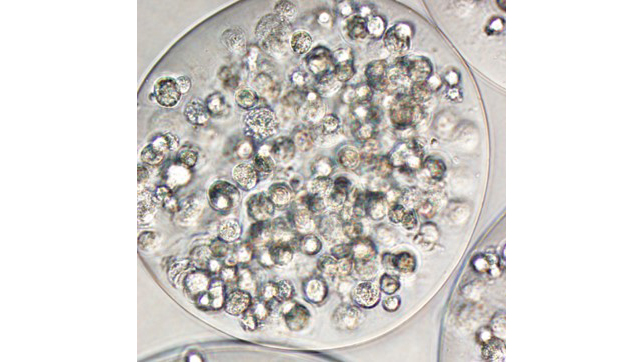
Stem cells
A major limitation to using stem cells to treat patients with cardiovascular diseases is the loss of transplanted cells almost immediately after they are delivered. The cells are washed out of the heart by blood flow, killed by the immune system, or fail to engraft. We are researching bio-compatible materials to support and direct the cells after they are implanted in the body. We focus on adult derived cell types easily obtained from most heart patients.
Publications
A complete list of our work can be found here.
Highlighted Publications:
- Sayegh MN, et al. Hydrogel delivery of purinergic enzymes improves cardiac ischemia/reperfusion injury. Journal of Molecular and Cellular Cardiology. 176. (2023); 98-109.
- Sayegh MN, Cooney KA, Han WM, Wang L, Strobel F, Hansen LA, Garcia AJ, Levit RD. Hydrogel Strategy to Augment Tissue Adenosine to Improve Hindlimb Perfusion. Arterioscler Thromb Vasc Biol. 2021.
- Hoang TN, Pino M, Boddapati AK, et al. Baricitinib treatment resolves lower-airway macrophage inflammation and neutrophil recruitment in SARS-CoV-2-infected rhesus macaques. Cell. 2021;184(2):460-475.e421.
- Xu, K., et al. (2019). "Adenosine from a biologic source regulates neutrophil extracellular traps (NETs)." Journal of Leukocyte Biology.
- Garcia JR, Campbell PF, Kumar G, Langberg JJ, Cesar L, Deppen JN, Shin EY, Bhatia NK, Wang L, Xu K, Schneider F, Robinson B, García AJ, Levit RD. Minimally Invasive Delivery of Hydrogel-Encapsulated Amiodarone to the Epicardium Reduces Atrial Fibrillation. Circulation: Arrhythmia and Electrophysiology. 2018;11.
- Shin EY, Wang L, Zemskova M, Deppen J, Xu K, Garcia AJ, Tirouvanziam R, Levit RD. Adenosine production by biomaterial supported mesenchymal stromal cells reduces the innate inflammatory response in cardiac ischemia reperfusion. JAHA, 2018.
- Garcia JR, Campbell PF, Kumar G, Langberg JJ, Cesar L, Wang L, Garcia AJ, Levit RD. A minimally invasive, translational method to deliver hydrogels to the heart through the pericardial space. JACC:BTS 2017.
Levit Lab Members

Rebecca Levit, MD
Dr. Levit came to Emory in 2007 after graduating from the University Of Pennsylvania School Of Medicine. She spent 7 years doing research and clinical training in cardiovascular disease. In 2014 she joined the faculty in the Division of Cardiology and is continuing her work on clinically translatable stem cell therapies for cardiovascular disease.
Gabriella Kabboul Massaad
Gabriella is a PhD student in the Wallace H. Coulter Department of Biomedical Engineering at both Emory University and Georgia Tech. Her research focuses on optimizing gene therapy strategies for cardiac applications.
Matthew Ensing
Matthew is a PhD student in the Wallace H. Coulter Department of Biomedical Engineering at both Emory University and Georgia Tech. His research focuses on innate inflammation in the heart utilizing transcriptomics.
Lucie Rosenberg
Lucie is a technician in the Levit Lab with expertise in histology and laboratory assays.
Lanfang Wang
Dr. Lanfang Wang is a technician in the Levit lab. She has been working in biomedical research for 20 years. She has experience with primary cell culture, cell encapsulation, immunofluorescence staining, western blot, and other techniques.
In the News
March 2024
Congratulations Sydney for winning the NAVBO travel award to the 2024 Gordon Research Conference on Lymphatic Vascular Diversity in Development, Tissue Homeostasis, Disease and Therapeutic Strategies.

February 2024
Congratulations Anamarie for winning the StARR award. Looking forward to having you in the lab and studying lymphatics in pediatric heart transplant.
December 2023
Had a great time at our lab group white elephant party. Thanks to Maegan for organizing.

May 2023
Congratulations to Dr. Deppen and Dr. Sayegh on graduation and earning their PhDs (pictured left and middle), as well as Anamaria for being one of the top three resident research day abstracts (pictured right)!
April 2023
Congratulations to Maegan for winning the 3-Minute-Thesis competition in the Molecular and Systems Pharmacology Program’s seminar course!

March 2023
The Levit Lab has their last lab meeting in Woodruff Memorial Research Building and moves to the new Health Sciences Research Building (II).
Congratulations to Maegan for presenting her research at the 20th annual DSAC research symposium!
February 2023
Congratulations to Michael for his paper being accepted to the Journal of Molecular and Cellular Cardiology!
January 2023
Congratulations to Juline for defending PhD thesis!

July 2022
Congratulations to Sydney and Maegan for presenting their poster abstracts at AHA BCVS 2022!
September 2022
Congratulations to Maegan and Gabriella for passing their oral qualifying exams!
October 2022
Congratulations to the lab for presenting their research at Emory University Department of Medicine Research Day!

June 2022
Congratulations to Juline for winning best Basic Sciences talk at Cardiology Research Day!
May 2022
Congratulations to Maegan for passing her written qualifying exam!
April 2022
Congratulations to Maegan for presenting her work at the 19th annual DSAC research symposium!
March 2022
Congratulations to undergraduate researcher Markus Cicka for defending his thesis with highest honors!
October 2021
Congratulations to Juline on winning the best oral presentation for basic science research at the Emory Department of Medicine Research Day!
August 2021
Congratulations to Maegan on being awarded the T32 Pharmacological Sciences Training Grant!
May 2021
Juline's paper was accepted into the Journal of Cardiovascular Transnational Research. Thanks to the Aronov Foundation and the CTSA for funding this work. Congratulations!
Congratulations to Dr. Michael Sayegh on successfully defending his thesis!
April 2021
Michael's paper was accepted into ATVB. Congratulations!
January 2021
Our first collaboration with Emory Primate Research Center and COVID-19 is published in Cell. Turns out neutrophils are important in viral infections, too.
Lab Alumni
- Maegan Brockman, PhD – Masonic Medical Research Institute, Kontaridis Lab, postdoctoral fellow
- Sydney Ginn, PhD – Cooley LLP, patent agent
- Triniti Scruggs, BS – Emory University, medical student
- Benoit Niclou, MD – Emory University, anesthesia resident
- Markus Cicka, BS - Washington University, MD, PhD student
- Kimberly Cooney, PhD - University of Arkansas, assistant professor
- Juline Deppen, PhD - MD Anderson Cancer Center, research associate
- Michael Sayegh, MD, PhD – Harvard University, resident in internal medicine
- Eric Shin, MD - Indiana University, assistant professor
- Kai Xu, MD, PhD - Central South University, China, professor
- Marina Zemskova, MS - University of Arizona, research technician

