The Lama Lab is a translational medicine research laboratory that combines clinical and basic science research to study and characterize phenotypes of Chronic lung allograft dysfunction (CLAD) and the cells and signaling pathways responsible for rejection and fibrogenesis.
Our team of researchers works every day to harness the power of translational research to furnish new understanding and promising solutions to combat allograft dysfunction, rejection, and fibrosis.
Our work includes developing robust animal models of rejection, studying mesenchymal stem cell signaling, and the transcriptional and translational regulation of their fibrotic function. These are just a few of the many avenues with which our team has chosen to fight against CLAD and towards a future where everyone can breathe a bit easier.
Our Inspiration
Here at the Lama Lab, we believe every person deserves to be able to breathe easily. For patients who experience chronic respiratory failure from end-stage lung diseases, however, lung transplantation remains one of the only viable solutions available to patients. But with a 10-year survival rate of just 20%, the field of lung transplantation and the anti-rejection treatments that follow have a ways to go before they can work for everyone.
Since 2003, our lab has worked ceaselessly to deepen our understanding of the mechanisms of rejection, to identify potential therapeutic targets and intervention points, and to play a role in developing new techniques and treatment options to patients all around the world.
It is our lab's ultimate mission to make lung transplantation a viable, safe, and effective option for any patient who may need it. For further information regarding our techniques, team, and ways to donate and get involved, please see our other pages--or reach out to us by email.
Our Work
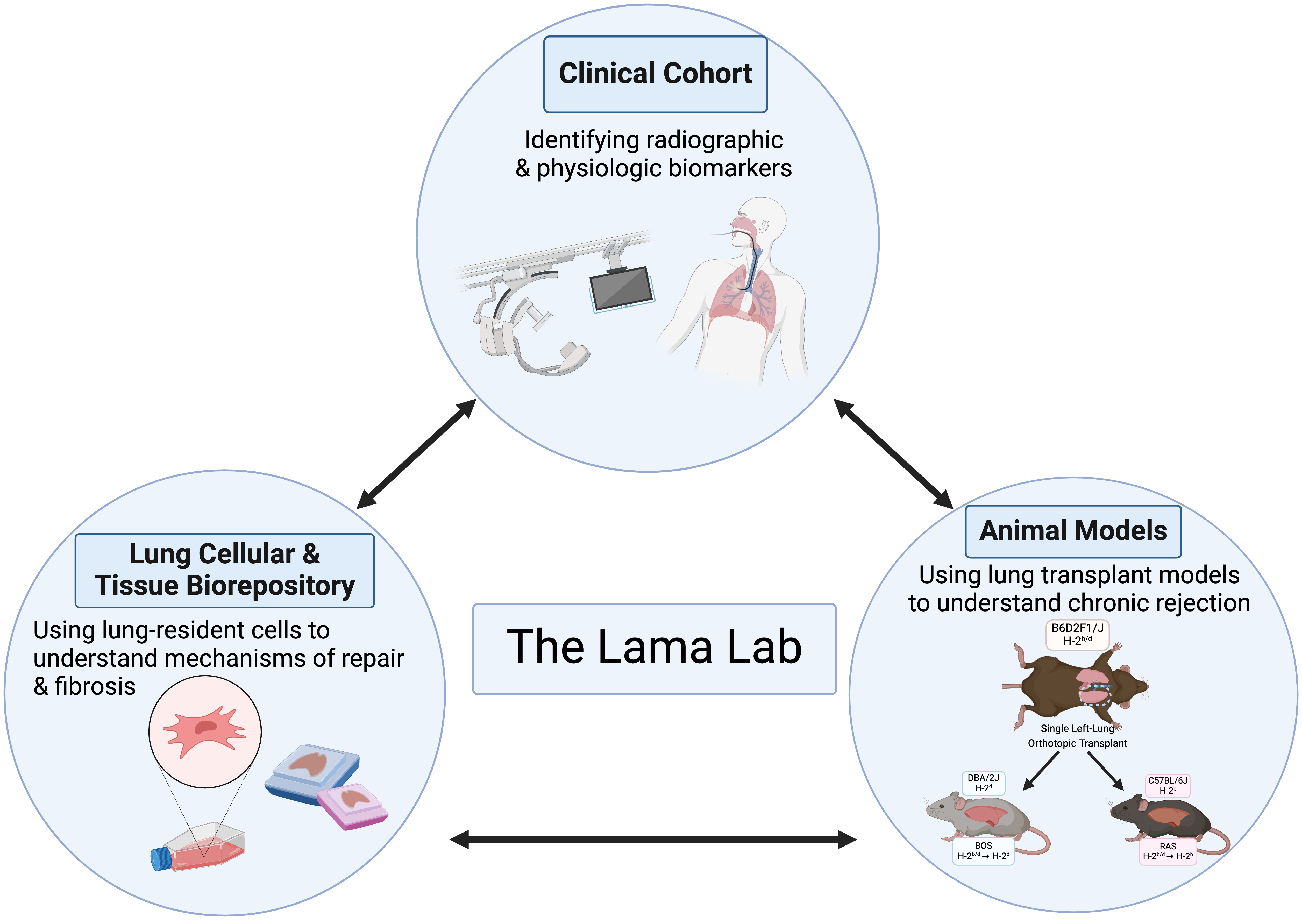
In the clinical arena, our primary focus has been improving lung allograft monitoring. Here, along with defining novel physiological parameters (spirometric decline patterns) and biological progenitor cell based biomarkers (mesenchymal colony forming units in bronchoalveolar lavage fluid), we have introduced novel radiographic imaging methodologies (parametric response mapping on HRCT) to monitor this population. We are now moving our biological biomarker discovery into the next phase by developing a marker panel of immune and fibroproliferation in such lavage fluid. A multicenter study investigating the application of our computed tomography voxel-wise methodology a novel imaging tool for lung transplant recipients is already underway.
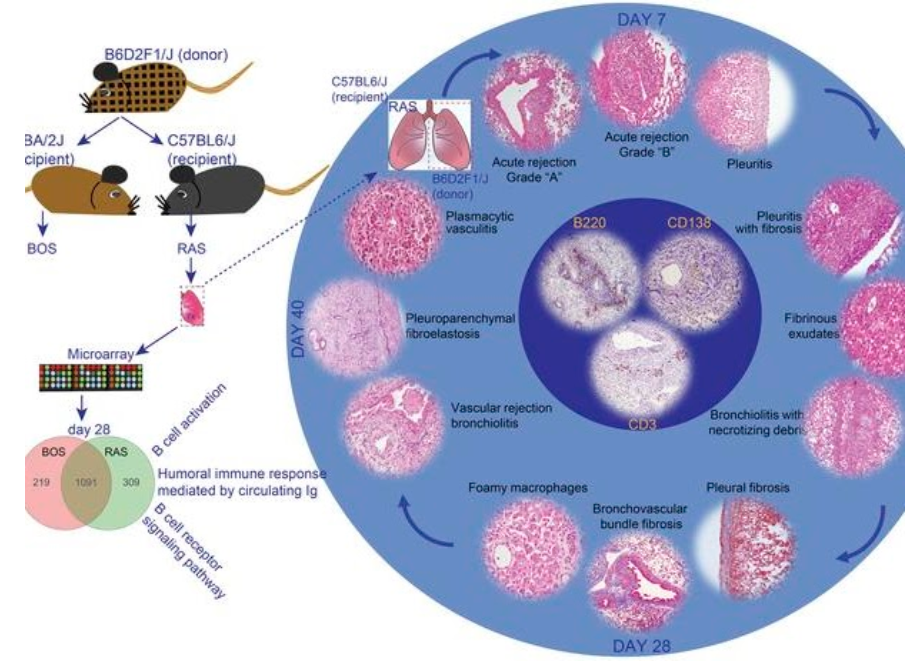
Another significant contribution of our work has been in animal models of lung transplantation. We were able to merge the in vitro mechanistic cellular studies with in vivo investigations by developing novel mouse orthotopic single lung transplant model of chronic rejection. We provided the scientific community with the first robust model of bronchiolitis obliterans post-lung transplantation by utilizing an F1 into a parent mouse. We have subsequently also developed a novel murine model for a more aggressive phenotype of chronic rejection after lung transplantation termed restrictive allograft syndrome (RAS). This recently published work demonstrated that skewing of immune responses determines the diverse allograft remodeling patterns and highlighted the need to develop targeted therapies for specific CLAD phenotypes.
In summary, our work transcends the fields of and allows collaborative work within arenas of immunology, developmental biology, regenerative medicine, and stem cells. In the planned next phase, we aim to merge our multi-faceted clinical phenotyping and our basic mechanistic pathway studies for discovering novel biomarkers of disease endotypes and patient-specific therapeutic targets.
Our Collaborations
Our Most Recent Publications
Results from randomized trial of pirfenidone in patients with chronic rejection (STOP-CLAD study).Volume: 43 Page(s): 1468 - 147709/01/2024 Authors: Combs MP; Belloli EA; Gargurevich N; Flaherty KR; Murray S; Galbn CJ; Vibha N Lama4
MNK-driven eIF4E phosphorylation regulates the fibrogenic transformation of mesenchymal cells and chronic lung allograft dysfunction.J Clin Invest Volume: 13408/15/2024 Authors: Walker NM; Ibuki Y; McLinden AP; Misumi K; Mitchell DC; Kleer GG; Lock AM; Vittal R; Sonenberg N; Garner AL; Vibha N Lama12
Genetic deficiency of the transcription factor NFAT1 confers protection against fibrogenic responses independent of immune influx.Am J Physiol Lung Cell Mol Physiol Volume: 326 Page(s): L39 - L5101/01/2024 Authors: Vittal R; Walker NM; McLinden AP; Braeuer RR; Ke F; Fattahi F; Combs MP; Misumi K; Aoki Y; Wheeler DS.
To view all publications from the Lama lab, please click HERE.
Contact Us
- Woodruff Memorial Research Building | 101 Woodruff Cir. Atlanta, GA 30322
- Emory Transplant Center | 1365 Clifton Rd NE Building B, Atlanta, GA 30322
- Division of Pulmonary, Allergy, Critical Care and Sleep Medicine | Emory University School of Medicine 100 Woodruff Circle Atlanta, GA 30322











