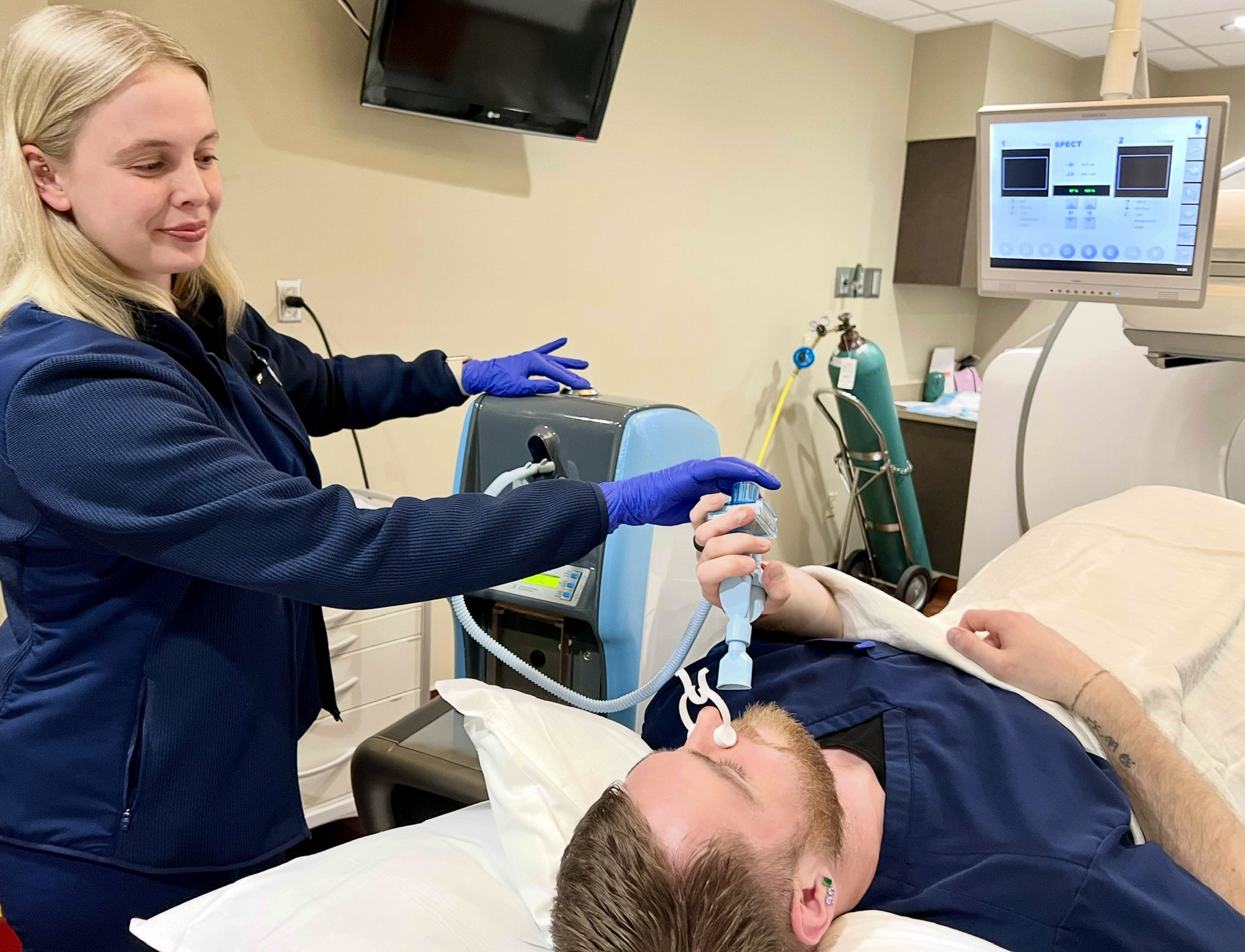
Emory Healthcare is the first system in Georgia to employ advanced imaging technology to better evaluate lung ventilatory function for diagnosis and treatment planning of conditions ranging from acute pulmonary embolism (new blood clots) to chronic thromboembolic pulmonary hypertension (old blood clots).
The Technegas® System diffuses technetium TC 99m labeled carbon inhalation aerosol (Technegas Aerosol®), a radioactive diagnostic nanoparticle, throughout the patient’s lungs as they undergo imaging called lung ventilation imaging. The radioactive nanoparticles behave like gas rather than particles, which help them to spread throughout the lungs, including into the tiny air sacs called alveolar spaces. Alveolar spaces are where the exchange of oxygen and carbon dioxide happens during lung ventilation (what we call breathing): oxygen flows in and carbon dioxide flows out of the lungs.
These images are then compared with perfusion images, which are obtained after injecting a radiotracer into the veins so images can show blood flow throughout the lungs. Comparing images of blood flow into and out of all areas of the lungs, including tiny capillaries in the alveolar spaces, with images of air flow in and out of the lungs, especially those small alveolar spaces, enables the nuclear medicine physician to determine if a life-threatening pulmonary embolism, or blood clot, may be present. For example, if an area of the lung shows no blood flow, but does show air flow, then there is a high likelihood of a blood clot, which is typically treated with anti-coagulation drugs.
Enhanced Patient and Technologist Safety
Technegas offers advantages over other types of inhaled radioactive imaging such as Xenon133 or technetium TC 99m DTPA. First, these radiotracers can yield hard to interpret images. More importantly, the method for administering them increases risk of transmitting COVID-19 and other communicable respiratory diseases. That transmission risk prompted providers including Emory to cease ventilation imaging and rely only on perfusion imaging during the pandemic.
The Technegas® System, on the other hand, is more self-contained and much safer to use with patients who have communicable respiratory infections such as COVID-19. The ability to reliably compare ventilation to perfusion imaging allows a more certain diagnosis of acute or chronic pulmonary embolism while protecting hospital personnel such as nuclear medicine technologists who must interact closely with patients during the imaging process.
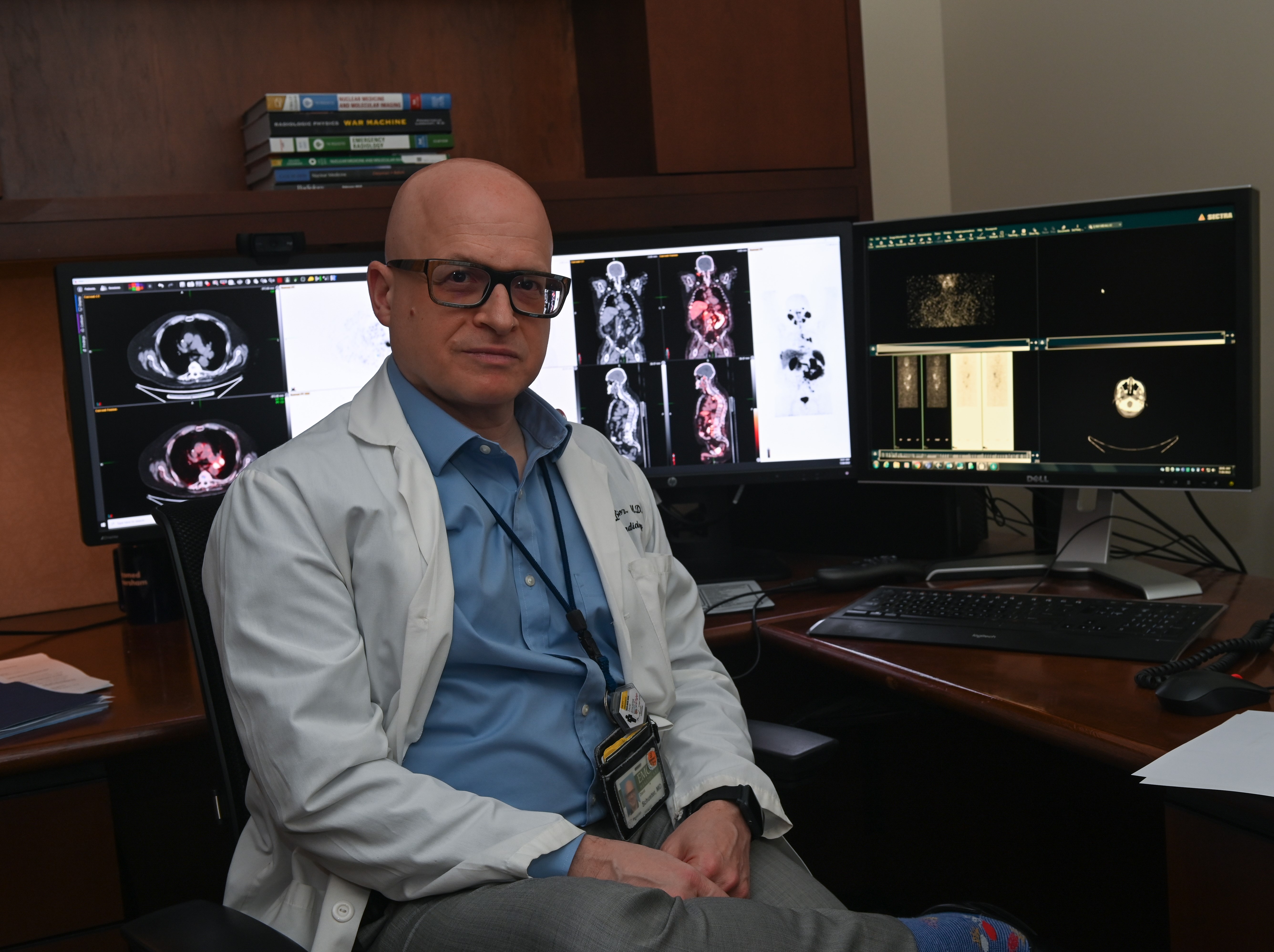
“Knowing with greater confidence which patients may be affected by a potentially deadly pulmonary embolus is a major step forward In caring for our patients,” says David M. Schuster, professor and director of the Division of Nuclear Medicine and Molecular Imaging in the Department of Radiology and Imaging Sciences of Emory University School of Medicine.
“During the COVID-19 pandemic we had to set this confidence aside as we could only perform the perfusion imaging but not ventilation imaging for fear of spreading the virus. This was like having a puzzle with a piece missing. As the pandemic waned, it was time to start thinking about performing ventilation imaging again, but we were unhappy with these old choices. Once Technegas was approved by the FDA, we saw this as a good perfect storm and our choice was simple. We now have a better-looking complete puzzle while protecting our staff and patients.”
Expanding Availability
This innovative imaging is available at Emory University Hospital Midtown and Emory Decatur Hospital and will eventually be available at all Emory facilities performing nuclear medicine studies.
The Technegas® System is a proprietary technology developed by Cyclopharm, a global radiopharmaceutical company delivering groundbreaking lung imaging solutions since 1986. It was approved in 2023 for clinical use by the U.S. Food and Drug Administration (FDA) for patients six years and older. Originally developed in Australia in the 1980s, it has become the preferred ventilation imaging agent in more than 60 countries. Emory is a proud early adopter in the United States of this important advancement in patient care.

