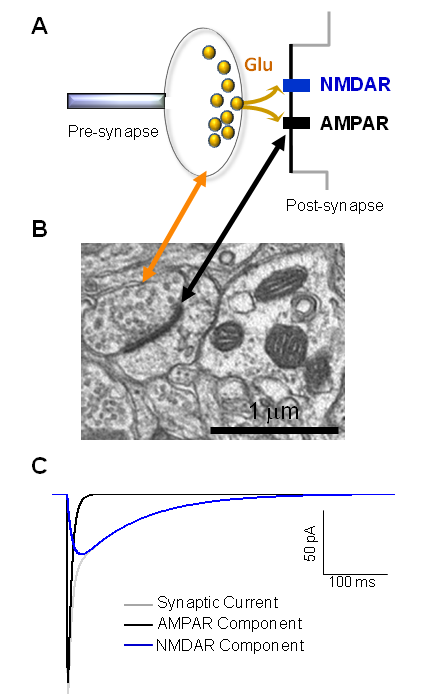
Neurons in the brain communicate by a variety of means, the most important of which is the process of synaptic transmission. Synaptic transmission involves a specialized connection between two neurons known as a synapse, which consists of a presynaptic terminal that releases a neurotransmitter onto a postsynaptic site that contains receptors for that neurotransmitter. These receptors bring about electrical or biochemical changes in the postsynaptic neuron, which alter its function and subsequent ability to mediate electrical signals to downstream neurons through additional synaptic contacts. Thought, cognition, and memory are all dependent on the pattern and strength of these synaptic connections, the most common of which fall into two main classes—excitatory and inhibitory fast synaptic transmission.
Fast excitatory synaptic transmission (see below) involves the release of glutamate from the presynaptic terminal, which diffuses across the narrow 30 nanometer synaptic cleft, where it encounters and binds to the glutamate receptor family of ligand-gated ion channels. At membrane potentials of neurons at rest (typically between -50 and -70 mV potentials), opening of a water filled pore through the transmembrane receptors by glutamate of the ligand gated ion channels allows cations (Na+, K+, and sometimes Ca2+) to enter the cell, depolarizing the membrane.
There are three main functional classes of glutamate receptors, AMPA, KA, and NMDA. These names are derived historically from synthetic and natural compounds first found to activate these receptor classes selectively:
- α-amino-3-hydroxy-5-methyl-4-isoxasolepropionic acid (AMPA) receptors,
- kainate receptors (KA),
- N-methyl-D-aspartate (NMDA) receptors.
This division was firmly established by identification of the genes that encode these receptors, which are subdivided based on sequence similarity into the same three classes
- GRIA which encode the AMPA receptor subunits (4 genes),
- GRIK which encode the kainate receptor subunits (5 genes),
- GRIN which encode the NMDA receptor subunits (7 genes).
The proteins encoded by these receptors assemble to form tetrameric protein complexes that form receptors with broadly shared architecture and features, yet distinct structure, functional, and pharmacological properties. These gene families reside on different neurons in different regions, each serving a different role.
In addition to these three main receptor types, genetic analysis also revealed two additional classes of genes that encode similar proteins, GRID (encoding delta subunits) and GRIN3 (encoding glycine-binding GluN3 subunits), each containing a pair of genes. These proteins, while thought to be important for brain function, are still poorly understood.
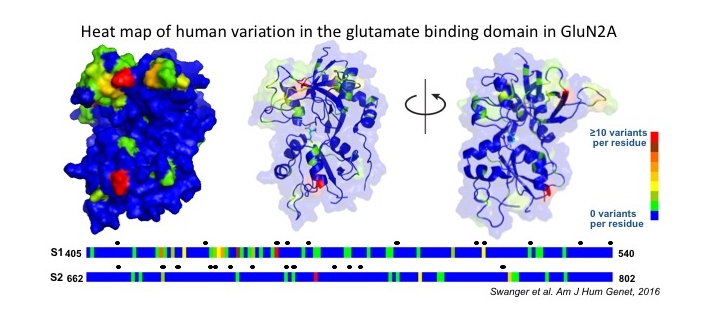
Further, a large family of accessory subunits that interact with glutamate-gated ion channels include transmembrane AMPA receptor regulatory proteins (TARPs) and other proteins such as Neto that interacts with kainate receptors. These accessory proteins associate with tetrameric glutamate receptors and have strong effects on receptor function. De novo mutations or rare variants in any member of this class of genes for the receptors or their accessory subunits could impact excitatory synaptic transmission, and thus health and behavior.