1. Molecular Mechanism of Action of New Drugs on Glucocorticoid and Mineralocorticoid Receptors
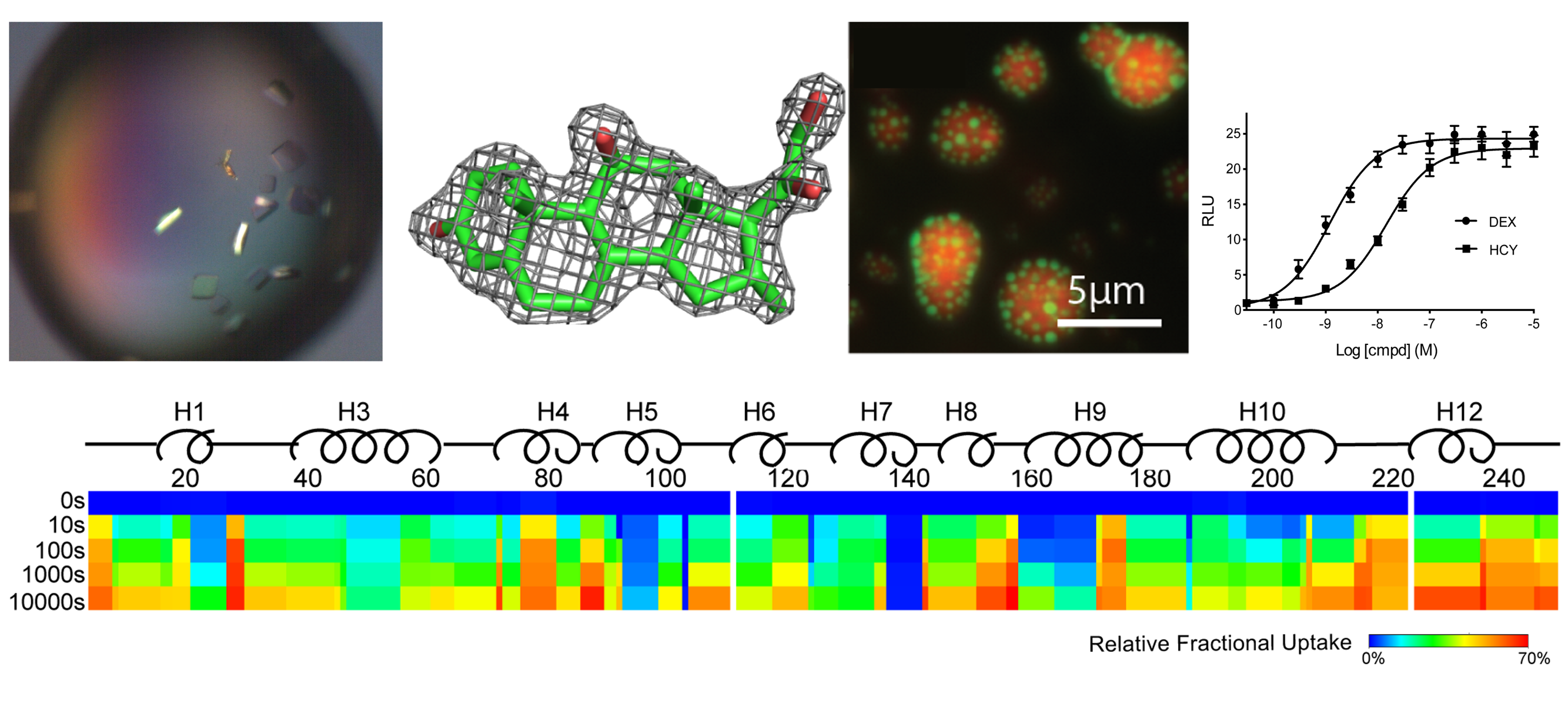
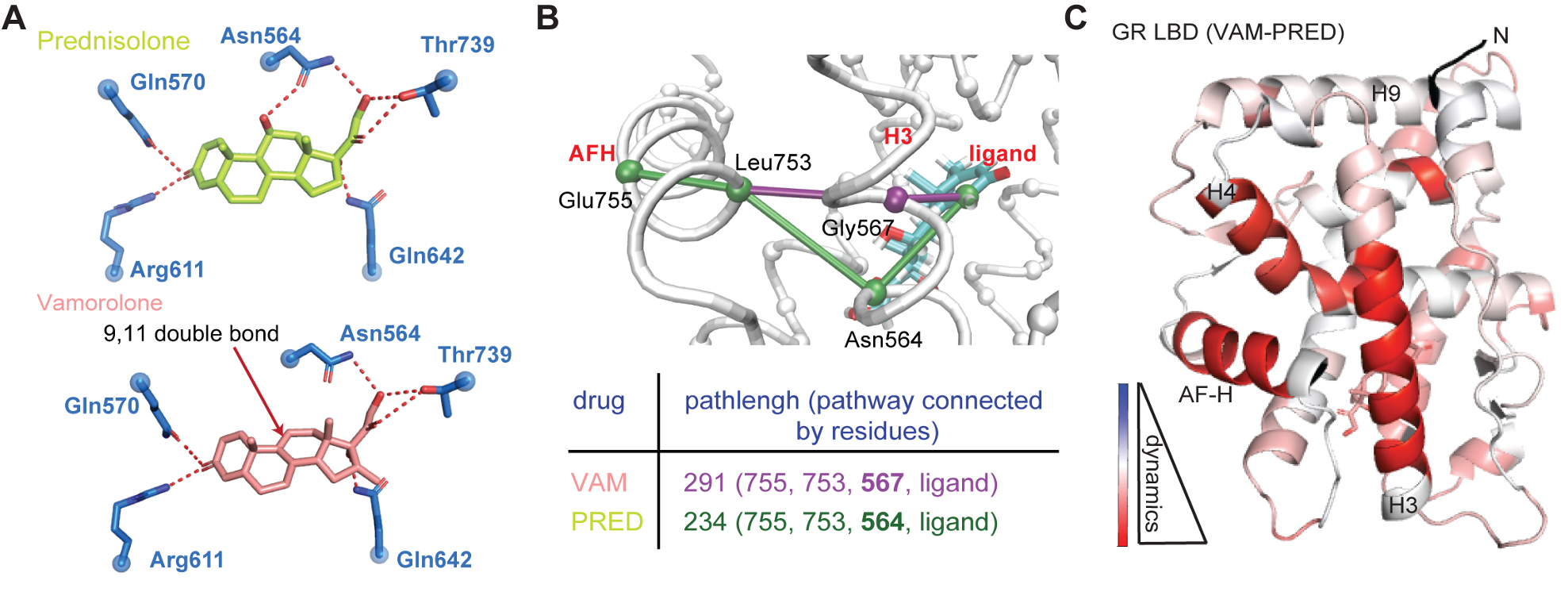
AGAMREE® (vamorolone) is an FDA-approved prescription medicine used to treat Duchenne muscular dystrophy (DMD), which is the most common and severe form of muscular dystrophy. In clinical trials, vamorolone has shown the ability to maintain muscle strength, similar to prednisolone therapy, but with fewer associated safety concerns, such as behavior changes and growth delays. Additionally, vamorolone has demonstrated cardiac protective efficacy in DMD mouse studies. Vamorolone acts as a selective glucocorticoid receptor (GR) agonist for anti-inflammation and a weak mineralocorticoid receptor (MR) antagonist for cardiac protection. Our research focuses on defining the structural and molecular mechanisms of action of vamorolone as it targets these steroid receptors. Our long-term goal is to use these mechanistic insights for developing new strategies to combat inflammatory and cardiovascular disorders.
2. Multifaceted Regulation of Mineralocorticoid Receptor Transcriptional Activities
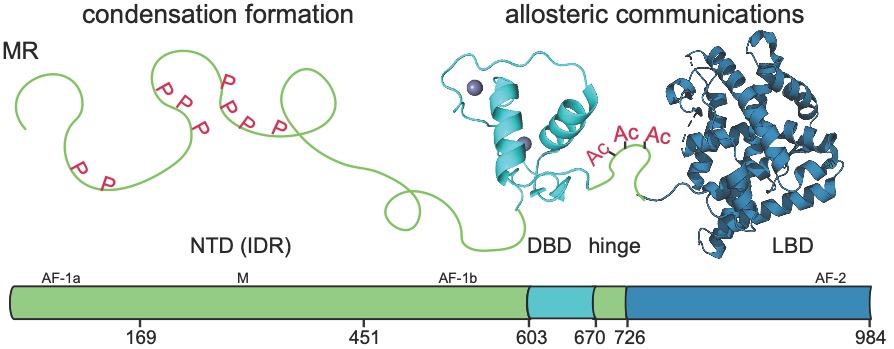
The steroid receptors share a highly disordered N-terminal domain (NTD), and two folded domains: a DNA-binding domain (DBD) and a C-terminal ligand binding domain (LBD), connected by a small unstructured hinge region. Despite numerous studies focusing on the individual LBD and DBD, how ligands elicit full-length NR activities remains elusive with massive knowledge gaps in the roles of NTD and allostery between DBD and LBD. The long-term goal of the laboratory is to develop the molecular-level understanding of the mechanism underlying the steroid receptor transcriptional activity by using mineralocorticoid receptor (MR) as an exemplary member. We will focus on 1) how NTD and hinge region regulate MR transcription by forming biomolecular condensates and 2) determine how the interdomain and intradomain allostery tune the MR-mediated transcription. An integration of biophysical approaches with structural biology and phase separation studies is essential to decipher this highly dynamic and disordered system and will be used here.
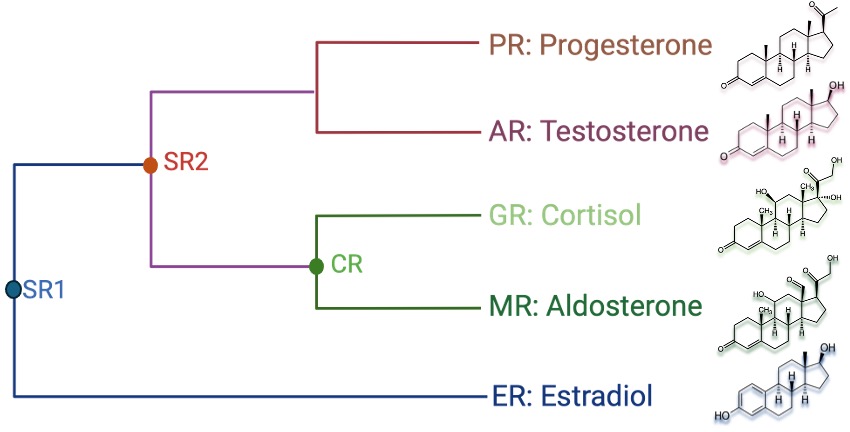
3. Evolutionary and Structural Insights into Ligand Specificity Switch in Steroid Receptors
In vertebrates, the mineralocorticoid receptor (MR) diverged from the glucocorticoid receptor (GR) through gene duplication approximately 450 million years ago. In terrestrial vertebrates, MR plays a crucial role in regulating sodium and potassium levels in response to aldosterone, whereas teleosts lack the ability to synthesize aldosterone. Progesterone, a steroid hormone involved in the menstrual cycle, pregnancy, and embryogenesis, acts as an MR antagonist in tetrapods but functions as an MR agonist in teleosts. The ability of these closely related receptors to elicit divergent responses to the same ligand is particularly intriguing. We leverage phylogenetically ancestral corticosteroid receptors (AncCR) to explore ligand specificity across extant hormones and drugs such as testosterone, progesterone, dexamethasone, and spironolactone. Our research aims to define the key residues and structural basis governing the specificity switch during evolution. Ultimately, our goal is to use these ancient proteins to better understand the cross-reactivity and off-target pharmacology of synthetic hormone drugs, combining structural and evolutionary biology approaches.