Classic molecular pharmacology is the study of how current therapeutics work to combat disease. While we remain committed to expanding our understanding of drugs’ mechanism of action, we’re also asking the all-important question of what’s next? Modern molecular pharmacology aims to lay the foundation for the development of next-generation medicines through the identification of novel drug targets and validation of new therapeutic strategies. This is often accomplished through the creative application of chemical methods to biological systems – chemical biology.
We are generally interested in kinases and phosphatases, which play opposing roles in maintaining balance in most signaling cascades and are often mutated or dysregulated in cancer. Our research is focused on 1) new kinase therapeutic strategies and 2) how phosphatases are regulated by protein-protein interactions.
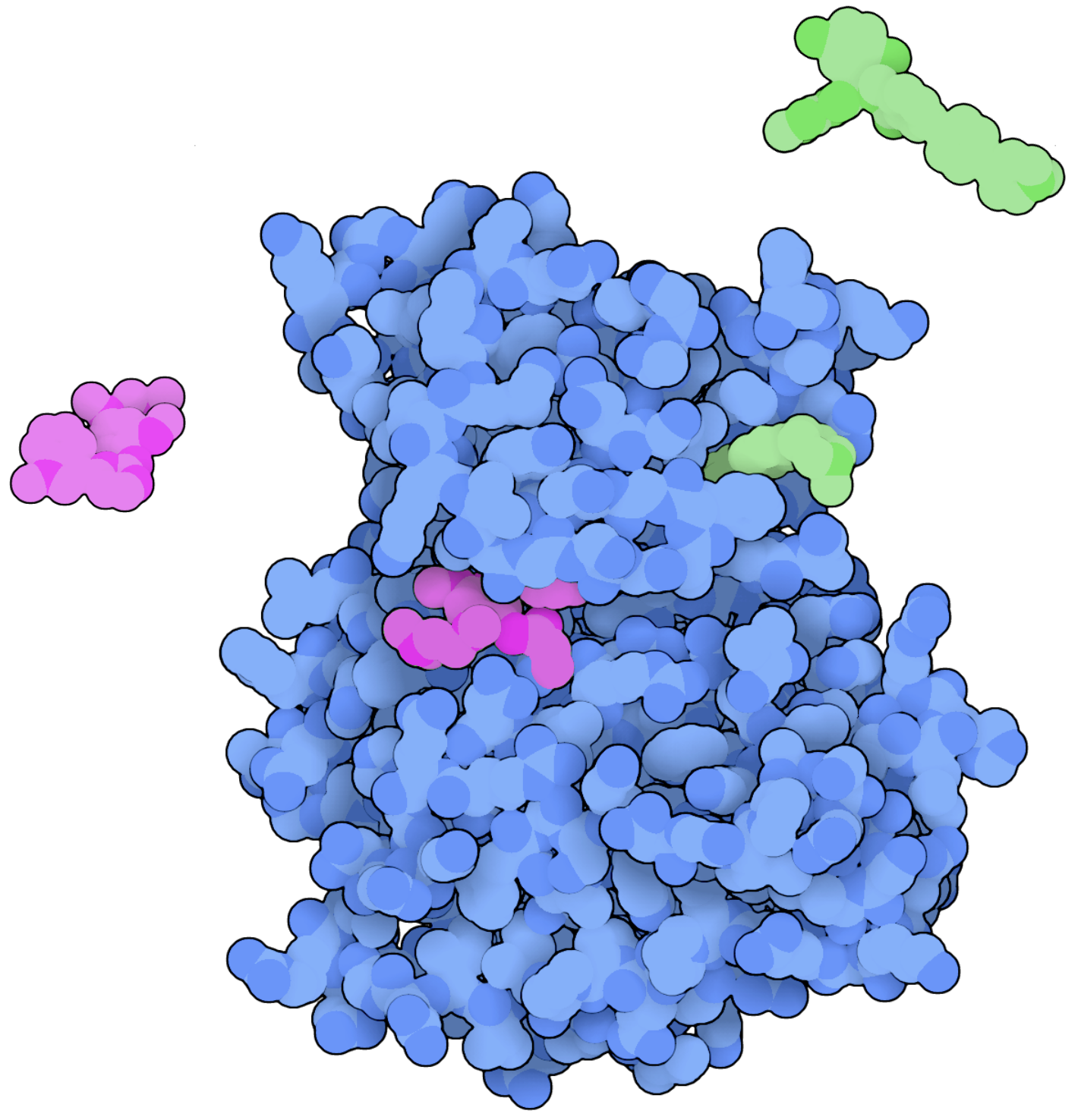
New Therapeutic Strategies for Kinases
Two is better than one, right? That is one of several questions we aim to answer when it comes to kinase therapeutics. While combination drug regimens are commonplace in cancer therapy, simultaneous “dual-targeting” or “double-drugging” of kinases is an emerging therapeutic strategy that we will explore in a number of different kinases (see Beyett, et al. Nature Communications, 2022).
We also aim to think outside the box, where the box is the kinase active site. Much like a factory machine, there’s many moving parts to a kinase, and figuring out all the places where you can jam a wrench to stop the machine can lead to the discovery of new types of inhibitors. Allosteric kinase inhibitors do just that, and we aim to identify new allosteric sites and mechanisms for kinases that we can target to better understand the pharmacological advantages and limitations of allosteric inhibition.
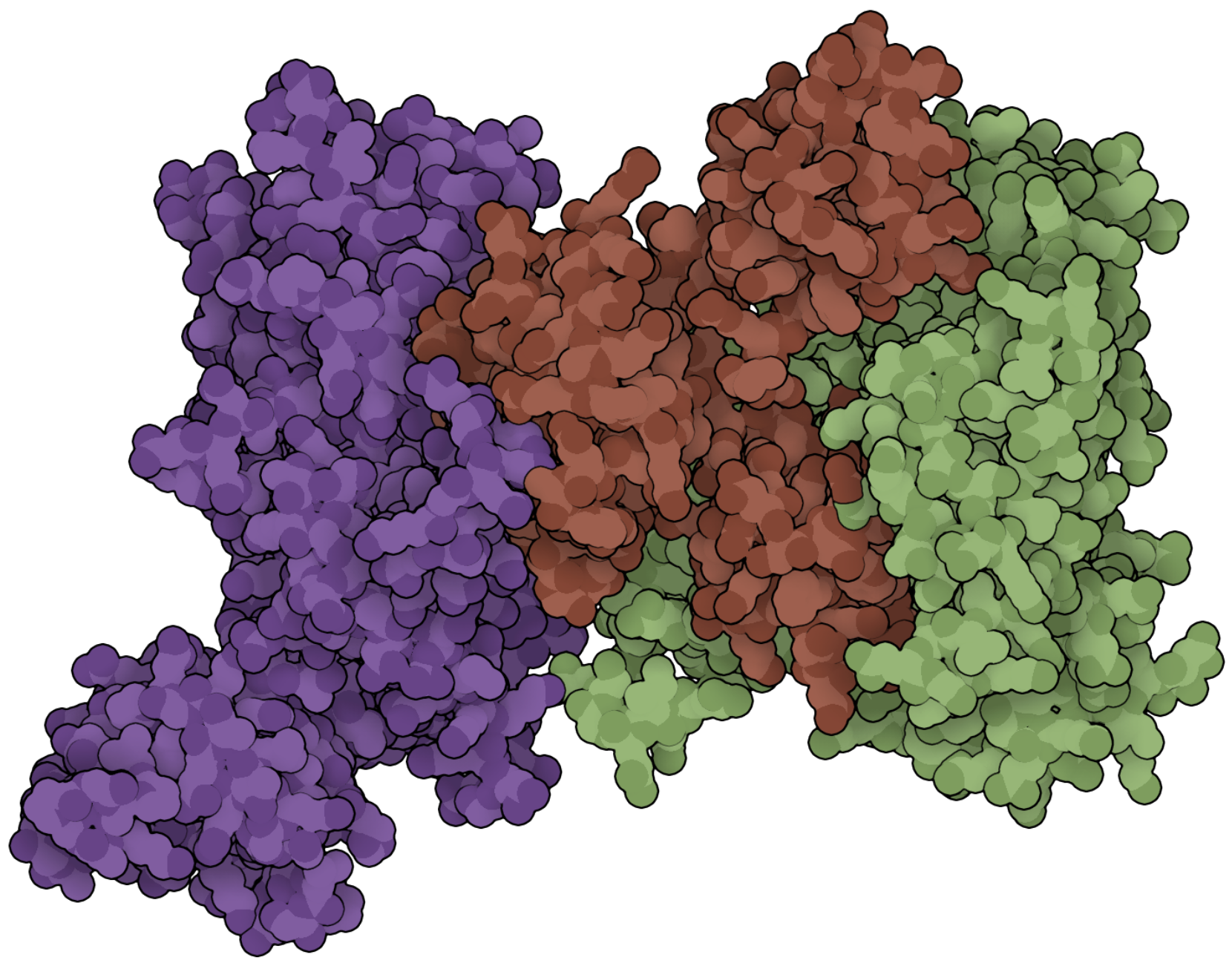
Probing Regulators of Kinase Signaling
Kinases and phosphatases. Yin and yang. Physiology requires balance and understanding this balance can open new therapeutic doors. We are studying the structure and function of novel regulators of phosphatases in a variety of important cellular signaling pathways to better understand how kinase signal transduction is regulated by these large protein complexes. Using chemical biology and high-throughput screening, we aim to develop chemical tools/probes with which to explore the potential of modulating these protein-protein interactions for therapeutic benefit.

