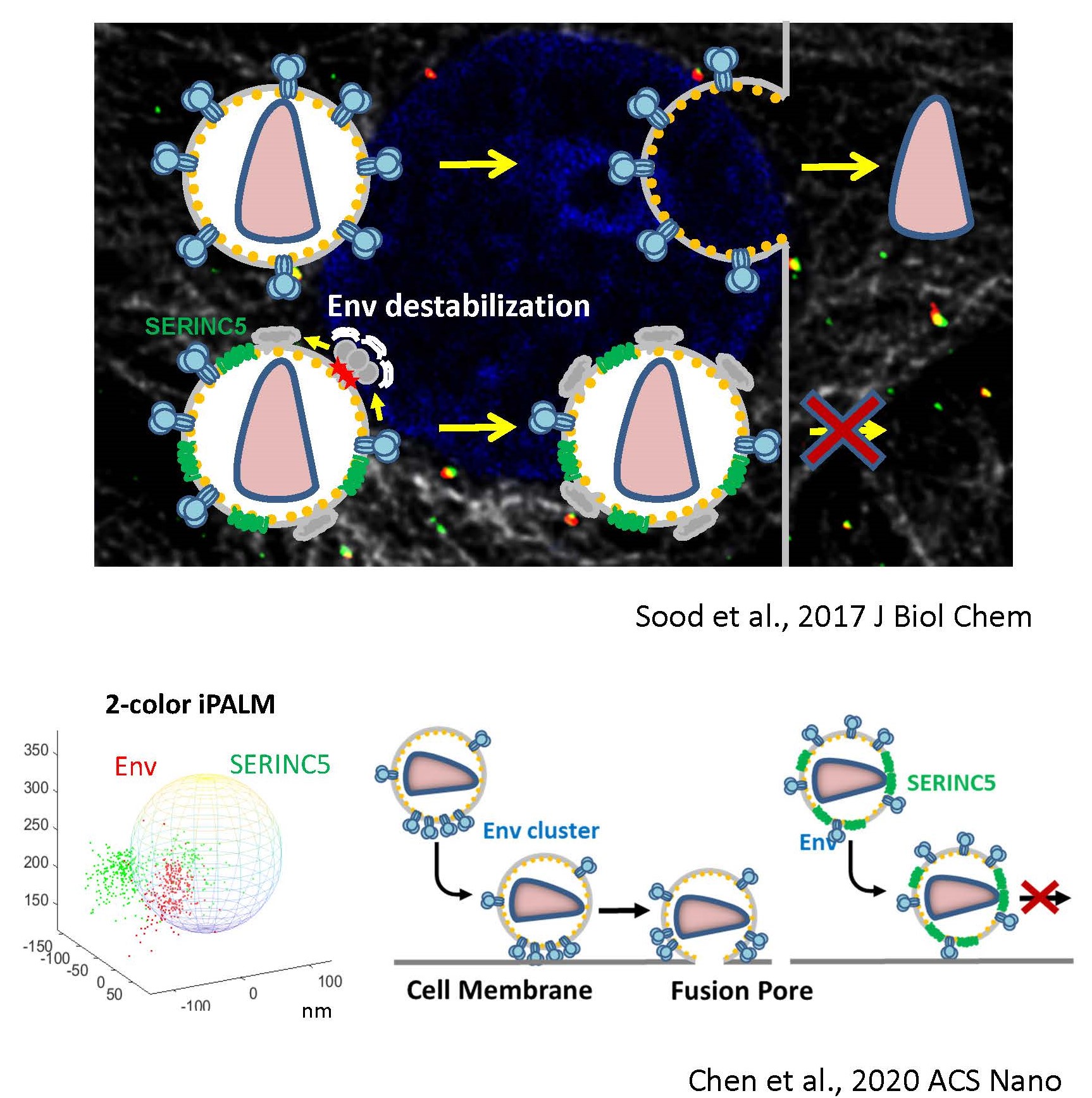
In the absence of HIV/SIV Nef expression, SERINC5 incorporates into budding virions and inhibits viral fusion, but the mechanism of restriction remains unclear. Envelope glycoproteins (Env) from different HIV-1 isolates exhibit a broad range of sensitivity to SERINC5. We have shown that SERINC5 incorporation alters Env conformation and sensitizes Env to neutralizing antibodies and fusion inhibitors. We also found that SERINC5 accelerates spontaneous inactivation of Env on virions (Figure 3). Using 2D- and 3D-superresolution imaging, we have observed that SERINC5 tends to disrupt functional Env clusters on mature HIV-1 particles. We have also implemented an assay to measure the transmembrane lipid asymmetry in single virions that allows to correlate SERINC5’s lipid scramblase activity to HIV-1 restriction. We are also interested in delineating the mechanism of HIV-1 restriction by the interferon-inducible host factor, MX2, that binds to viral capsid.