Interplay between ubiquitylation and protein methylation in KLF4-mediated genome stability and breast carcinogenesis
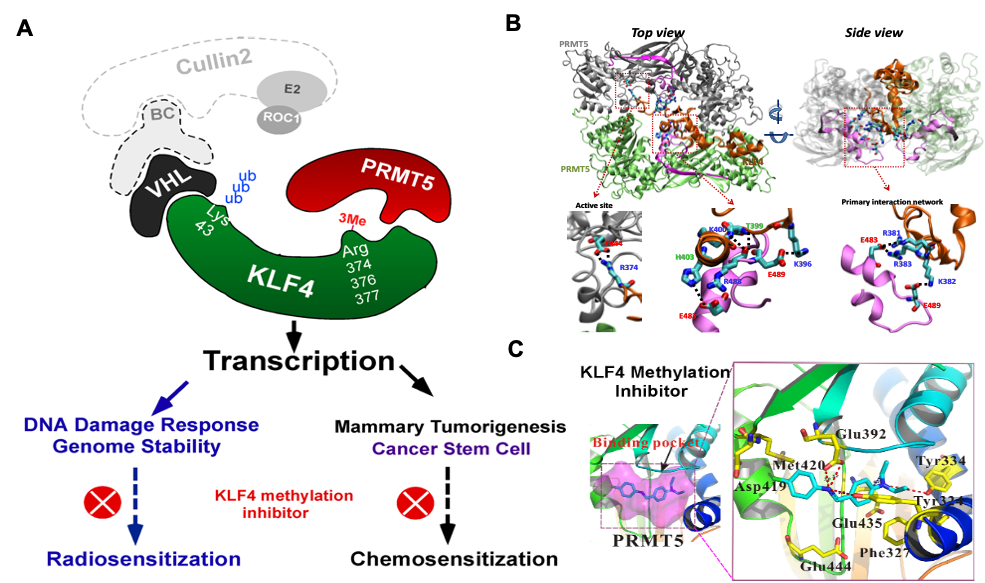
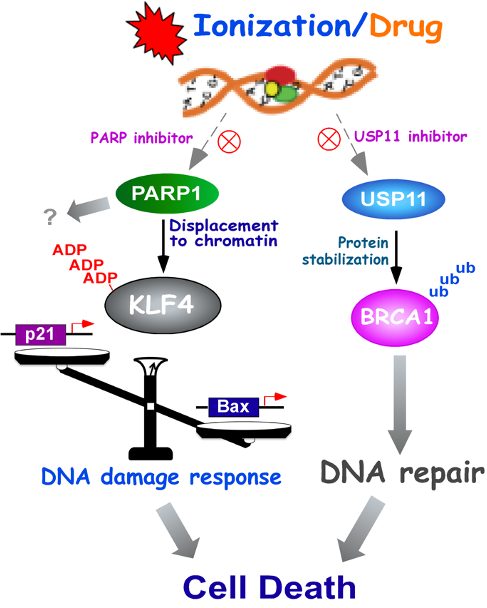
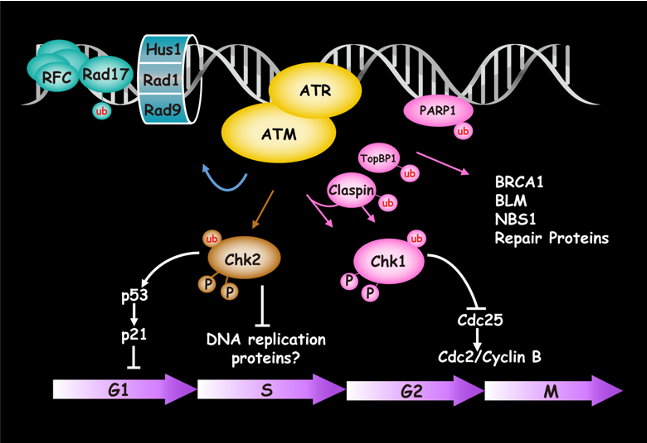
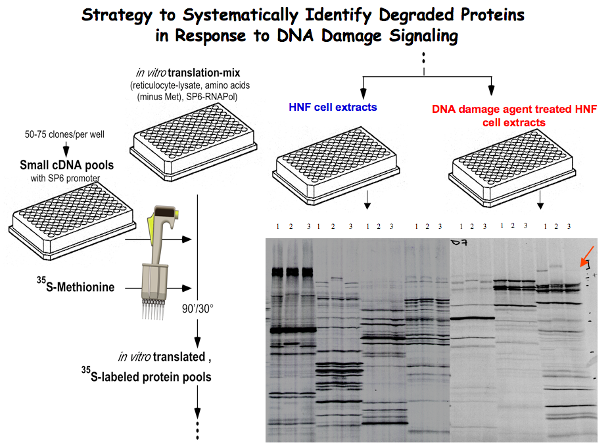
Kruppel-like factor 4 (KLF4) is an important regulator of cell-fate decision, including DNA damage response, inflammation, apoptosis, and stem cell renewal. Its critical impact in breast cancer formation was recently uncovered by the TCGA (The Cancer Genome Atlas) study as well as human breast cancer specimen tissue array. Surprisingly, recent studies have sketched an ambivalent nature for KLF4 in tumorigenesis as either a tissue specific tumor suppressor (in colorectal cancer) or oncogene (in breast cancer), although the underlying mechanism as to how it switches functions remains unclear. In addition, how KLF4 is regulated in response to various environmental factors such as DNA damage signal remains largely unknown. To explore the mystery, we have purified KLF4 protein complexes followed by identification of its physiological binding partners using mass spectrometry. This effort has led to the identification of several KLF4 physiological interacts including VHL (a ubiquitin ligase), PRMT5 (an arginine protein Methyltransferase) and PARP1 (poly (ADP-ribose) polymerase 1). Results from our initial characterization suggest that precise KLF4 protein levels were determined by VHL/VBC and PRMT5. While KLF4 is targeted by VHL/VBC for ubiquitylation and degradation, PRMT5-mediated methylation antagonizes KLF4 ubiquitylation thereby stabilizing KLF4. Given that KLF4 is a pivotal cellular-fate factor after exposure to DNA damage, we are now addressing how the crosstalk between ubiquitylation and methylation dictates DNA damage response by promotion of p21 and inhibition of Bax. In addition, using a protein structural and computational modeling analysis, we are elucidating the mechanisms of how PRMT5 catalyzes KLF4 methylation and how KLF4 methylation counteracts KLF4 ubiquitylation for stabilization. The ultimate goal is to develop small molecule inhibitors that could intercept PRMT5-mediated KLF4 methylation, which could be valuable for anti-breast cancer chemotherapy. This project is funded by NIH/NCI R01 grant (CA250110).