
Emory researchers are on the cusp of seeing in real-time how brain diseases like Alzheimer’s and autism spectrum disorder affect a patient’s brain function. That window into the brain in turn will let them more effectively develop medications to treat the more than 10 million people in the US living with these still-baffling conditions.
Two R01 grants from the National Institute on Aging and the National Institute of Mental Health will fund development of novel positron emission tomography (PET) imaging probes for Alzheimer’s disease and autism spectrum disorder. The awards will provide a total of $9.6 million over five years to support multidisciplinary teams collaborating through the new Positron Emission Tomography Imaging Center and the Radiopharmaceutical Discovery Program, both located in the Department of Radiology and Imaging Sciences of the Emory University School of Medicine. Collaborating departments include Pharmacology and Chemical Biology, Neurology, and Psychiatry.
“We are truly honored to receive this federal support to advance our understanding of Alzheimer’s disease and autism spectrum disorder by using novel PET imaging technologies,” says principal investigator Steven Liang, PhD, an associate professor in the Department of Radiology and Imaging Sciences. In addition to directing the Radiopharmaceutical Discovery Program, Liang also directs the Emory PET Imaging Center and is the Endowed Director of Radiochemistry, Cyclotron Facility and Radiopharmacy in Emory’s Center for Systems Imaging.
“Selective PET probes bring up target-specific information in living subjects, and that information reveals disease stage and progression,” he explains, “By collaborating with stellar Emory faculty members in translational brain research, we can more effectively and quickly advance clinical investigation and drug discovery to help the millions of people who are living with Alzheimer’s disease as well as other forms of dementia.”
The Power of PET Imaging
PET is a non-invasive imaging technique that creates images of biochemical processes as they occur in the living body, or in vivo. Radioactive molecules, or ligands, are injected into the body and the distributions of those molecules are measured using a PET scanner. The unique detection physics of PET leads to images that quantitatively assess metabolism, protein binding, receptor density, blood flow, cellular synthesis, and other biological processes specific to human diseases. Scientists call these radioactive ligands PET probes because of their ability to plumb the depths of biological function.
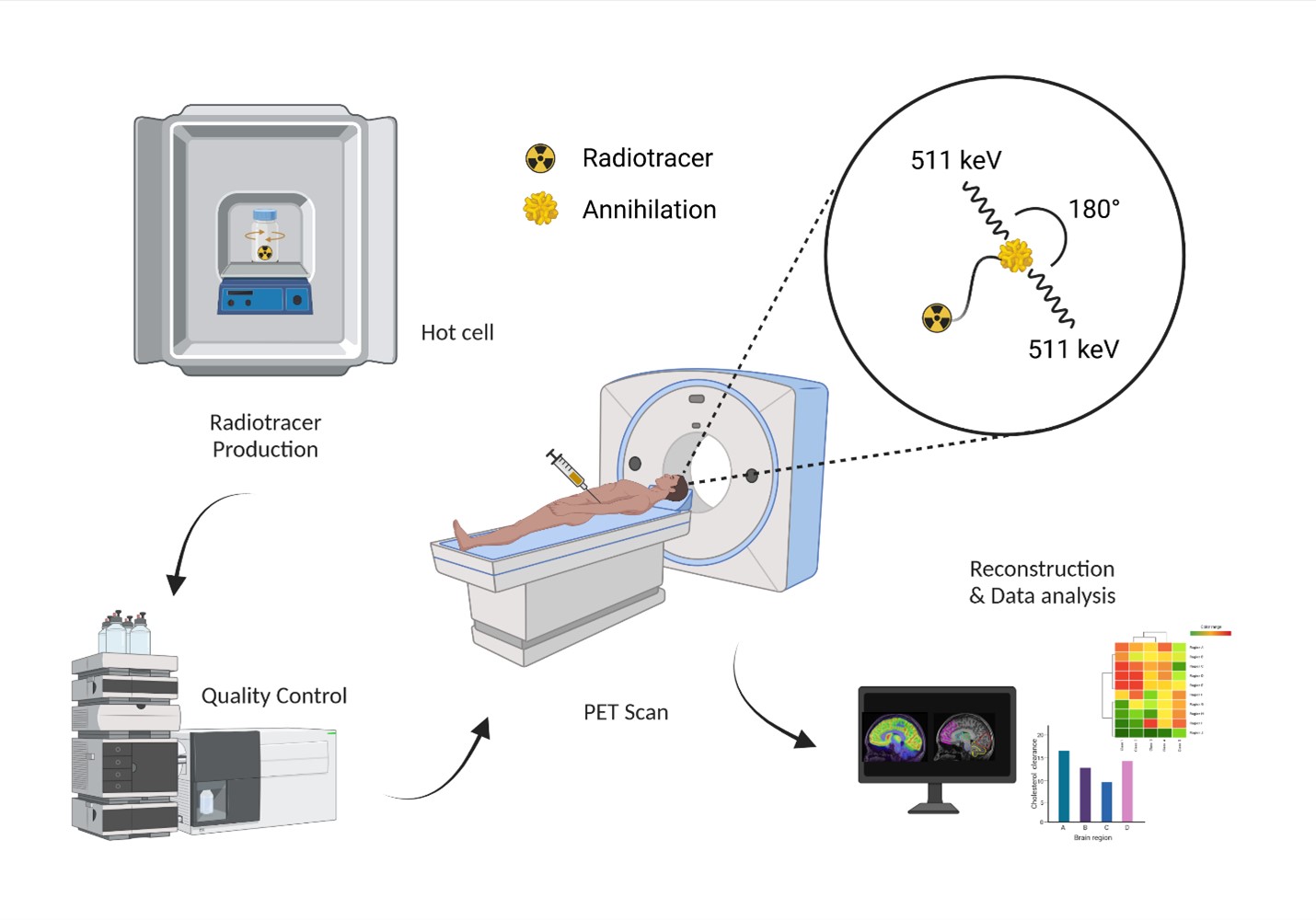
PET Probes for Alzheimer's Disease
Previous research implicates dysfunction of the GluN2B subunit of the N-Methyl-D-aspartic acid receptor (NMDAR) in the brain as contributing to the physiopathology of neurodegenerative diseases such as Alzheimer’s and related forms of dementia. In the R01-funded Alzheimer’s-related research, Liang and colleagues will develop potent and selective GluN2B PET ligands to assess the biological processes of these neurodegenerative diseases. Once successful, the next step is to advance the ligand for potential clinical translation, that is, to develop the ligand to be used with PET imaging to assess the effects of novel neurotherapeutics being developed to treat neurodegenerative diseases like Alzheimer’s, which the CDC says affects 6 million adults.
The project is a multidisciplinary collaboration with co-investigators Stephen F. Traynelis, PhD, professor, and Hongjie Yuan, MD, PhD, associate professor, both from the Department of Pharmacology and Chemical Biology, and Allan Levey, MD, PhD, Robert W. Woodruff Professor of Neurology, Goizueta Foundation Endowed Chair for Alzheimer’s Disease Research and Director of the Goizueta Alzheimer’s Disease Research Center.
PET and Autism Spectrum Disorder
As part of the autism-related R01 project, Liang steers a cross-center and cross-departmental collaboration with Larry Young, PhD, professor of psychiatry and behavioral sciences and Director of the Center for Translational Social Neuroscience at the Emory National Primate Center to develop novel PET probes targeting vasopressin, a key receptor researchers identify as responsible for some autism spectrum disorder (ASD) pathophysiology.
“A suitable vasopressin-targeted PET probe could substantially improve our understanding of the arginine vasopressin receptor-signaling pathway in ASD, which is otherwise inaccessible for biochemical analysis, particularly in humans,” he explains.
The team will develop a specific PET probe as the first translational imaging tool for autism research. Non-invasive quantification of vasopressin in the living brain by PET imaging would make it possible to assess new neurotherapeutics for treating autism spectrum disorders in real time: showing how and where and by what magnitude they positively affect the brain. Such breakthroughs cannot come fast enough given that the CDC reports one in 44 children are diagnosed with ASD and about 5.5 million adults currently are living with ASD.
From Promise to Profound Impact
“This work has the potential to be game-changing,” says Elizabeth Krupinski, PhD, professor & vice chair for research in the Department of Radiology and Imaging Sciences. “Dr. Liang’s projects involve drug discovery, radiochemistry, and translational PET imaging studies in multiple species, from rodents to nonhuman primates to humans. We are very excited to support this fruitful collaboration, a result of our Team Science approach, and to stimulate cross-departmental interactions to translate basic science discoveries into humans.”
Vikas Sukhatme, MD, ScD, dean of Emory School of Medicine, agrees: “These projects are great examples of Emory’s expertise in bench-to-bedside translational research, research with substantial potential for improving patient care. The work will take place in our new Health Sciences Research Building (HSRB)-II, supported by our Center of Systems Imaging Core, proving what world-class research facilities can to do improve our understanding and treatment of complex conditions.”
Moreover, Emory has exceptional strength in basic research on receptor biology, with leading experts studying the targets for which Liang is developing imaging probes. This coupled with the long tradition of collaborative multi-scale research efforts provides a solid foundation for this game-changing research program.
Emory: A National Leader in Advanced Imaging Innovation
Emory is a natural home for this work, says Amit Saindane, MD, MBA, professor and interim chair in the Department of Radiology and Imaging Sciences. “Emory Radiology has a stellar record when it comes to developing advanced imaging technology, radiopharmaceutical discovery, and translational research.”
That record includes the FDA-approved Emory Cardiac Toolbox™. Developed by Ernest Garcia, PhD, emeritus professor of radiology and imaging sciences, it is one of the most widely applied cardiac imaging systems in the world.
Emory also is a pioneer in imaging theragnostics for prostate cancer. Emory Radiology professor Mark Goodman discovered and holds the patent for the radiopharmaceutical fluciclovine (18F)
Marketed by Blue Earth Diagnostics as Axumin®, it now is widely used to detect recurrent prostate cancer. Emory Radiology’s David M. Schuster, MD, professor and director of the Division of Nuclear Medicine and Molecular Imaging, led the clinical trials required to gain Food and Drug Administration (FDA) approval of the radiotracer in 2016. More recently, Dr. Schuster and colleagues have validated the radiotracer’s value in guiding clinical decision-making for patients with recurring prostate cancer. Additional research continues.
Saindane sees this next wave of discovery as equally promising. “The novel PET imaging tools developed by Dr. Liang and his research team show great potential for furthering our understanding of these devastating neurodegenerative and neurodevelopmental diseases. We hope to advance these innovative imaging technologies so they then can be used in research testing novel neurotherapeutics for treatment.”

