Treatment guided by molecular imaging technology invented and developed by Winship researchers shows improved disease-free survival rates for men who have undergone surgical removal of their prostate, according to findings published in the The Lancet.
May 10, 2021
Treatment guided by molecular imaging technology invented and developed by researchers at Winship Cancer Institute of Emory University shows improved disease-free survival rates for men who have undergone surgical removal of their prostate, according to findings published in the medical journal The Lancet.
The Emory Molecular Prostate Imaging for Radiotherapy Enhancement, or EMPIRE-1, was led by Winship radiation oncologist and prostate cancer specialist Ashesh B. Jani, MD, MSEE, FASTRO, and Winship nuclear radiology specialist David M. Schuster, MD, FACR.
The manuscript, "EMPIRE-1: Randomized Trial Comparing Conventional- vs Conventional plus Fluciclovine (18F) PET/CT Imaging-Guided Post-Prostatectomy Radiotherapy for Prostate Cancer" was published online in The Lancet on May 7.
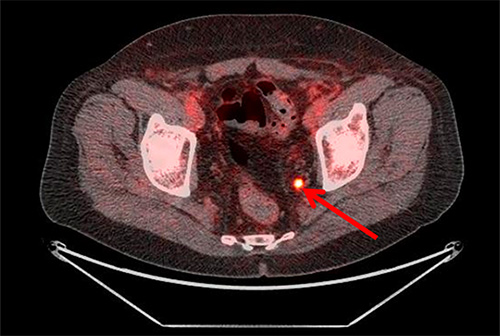
The phase 2-3 study used the radiotracer fluciclovine (fluorine-18, or 18F) which was invented by a multidisciplinary team at Emory and is now commercialized by Blue Earth Diagnostics.
The randomized trial enrolled 165 patients whose cancer recurred after having their prostates removed. One group received radiation therapy using conventional imaging while the other received treatment using imaging with the fluciclovine PET radiotracer. The study found that 75.5 percent of those whose treatment integrated fluciclovine PET were disease-free after three years, compared to 63 percent for those where only conventional imaging techniques were used to plan treatment. The increased failure-free survival rate persisted after four years of follow up.
Fluciclovine was originally developed by Mark M. Goodman, PhD, Winship researcher and professor of radiology and imaging sciences at Emory University School of Medicine, and colleague Timothy Shoup, PhD, currently at Massachusetts General Hospital. In 1999, Goodman, Shoup, and Winship neurosurgeon Jeffrey J. Olson, MD, published a study on the use of fluciclovine for brain tumor imaging.
More than a decade ago, researchers decided to see if fluciclovine could also work for prostate cancer. Funded by a $2.2 million grant from the National Institutes of Health (R01 CA 169188), Jani and Schuster began the EMPIRE-1 trial (NCT 01666808) in 2012 to explore the role of advanced molecular imaging with fluciclovine in men who had undergone prostatectomy.
The technology was licensed to Blue Earth Diagnostics in 2014, and in 2016 the U.S. Food and Drug Administration approved the use of fluciclovine to diagnose men with recurrent prostate cancer with elevated blood levels of prostate-specific antigen after previous treatment. It is the first FDA-approved fluorinated PET radiotracer (trade name Axumin) for prostate cancer staging.
"The decision to offer post-prostatectomy radiation is complex, because conventional imaging can leave unanswered questions on the best approach for treatment planning," says Jani. "This research has found that integrating advanced PET imaging using fluciclovine allows us to do a better job of selecting patients for radiation therapy, guiding radiation decisions and planning, and ultimately, keeping our patients’ cancer under control."
"The question that we wanted to answer in this study was whether the treatment plan informed by fluciclovine PET imaging had a positive effect in the lives of patients," says Schuster. "The significant results of the EMPIRE-1 trial confirm that it does."
The findings were featured at the American Society for Radiation Oncology's annual meeting last October.
Jani and Schuster are also leading another NIH-funded trial, EMPIRE-2 (NCT 03762769), to investigate a newer type of advanced molecular imaging, PSMA or prostate specific membrane antigen PET. The new radiotracer targets a receptor on the surface of prostate cancer cells. Investigators will assess how this newer type of PET scan compares with fluciclovine in improving cancer control for prostate cancer patients with recurring cancer.

