The general research interests of my laboratory include investigating neuroinflammatory responses in the diseased brain, in particular we are interested in examining the context by which activation and recruitment of myeloid cells subsets are beneficial or harmful after acute brain injury and chronic neurologic disease. Utilizing a number of approaches including genetically modified mouse models, chemoconvulsant rodent models of status epilepticus and epilepsy, immunohistochemistry, microscopy, cell culture, and gene induction analysis we aim to identify immune-related targets to ameliorate the deleterious consequences of brain disease.
The principle focus of my current research portfolio is to establish brain-invading monocytes as a viable target to alleviate neuronal damage and morbidity after the seizures of status epilepticus and glean insights into the manner by which monocyte brain entry is pathogenic. The anticipation is that these studies will address fundamental unanswered questions about how the peripheral immune system can impact the brain inflammatory environment and elucidate how brain-invading monocytes contribute to neuropathology, neuroinflammation, and morbidity. I have recently received a fundable score on a five-year grant from the National Institutes of Neurological Disease and Stroke to examine the following:
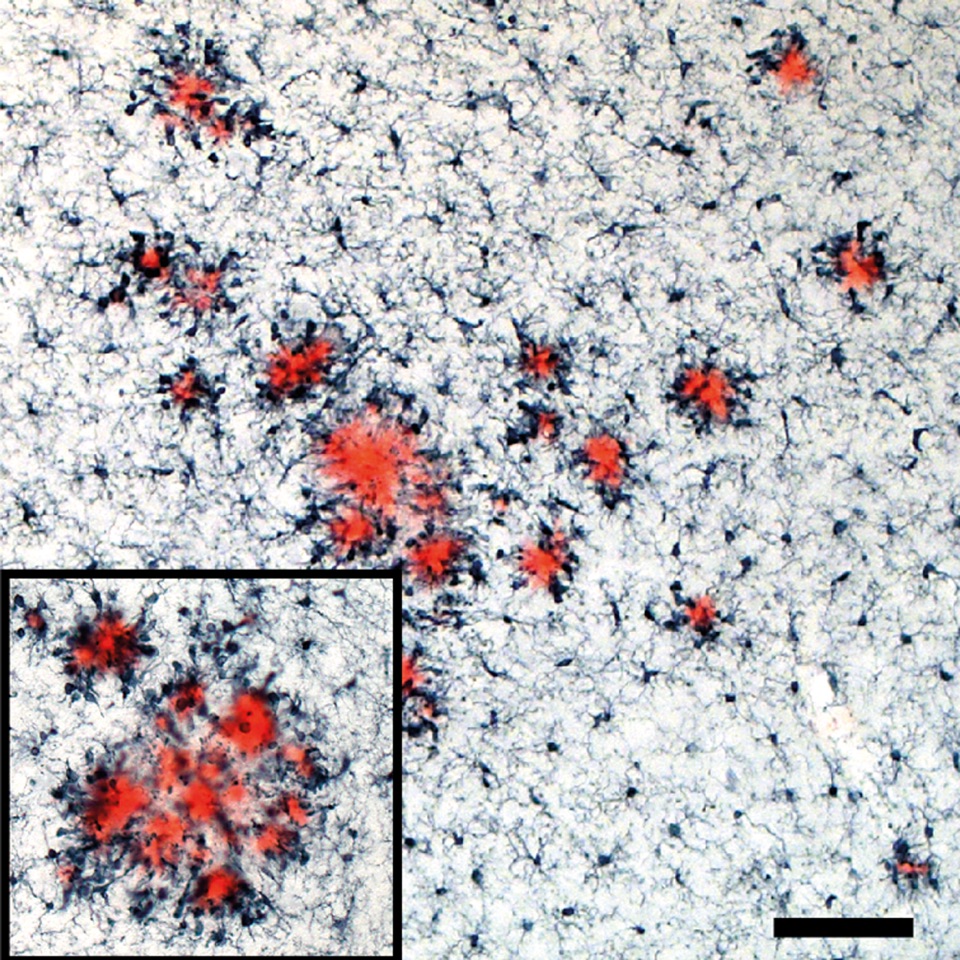
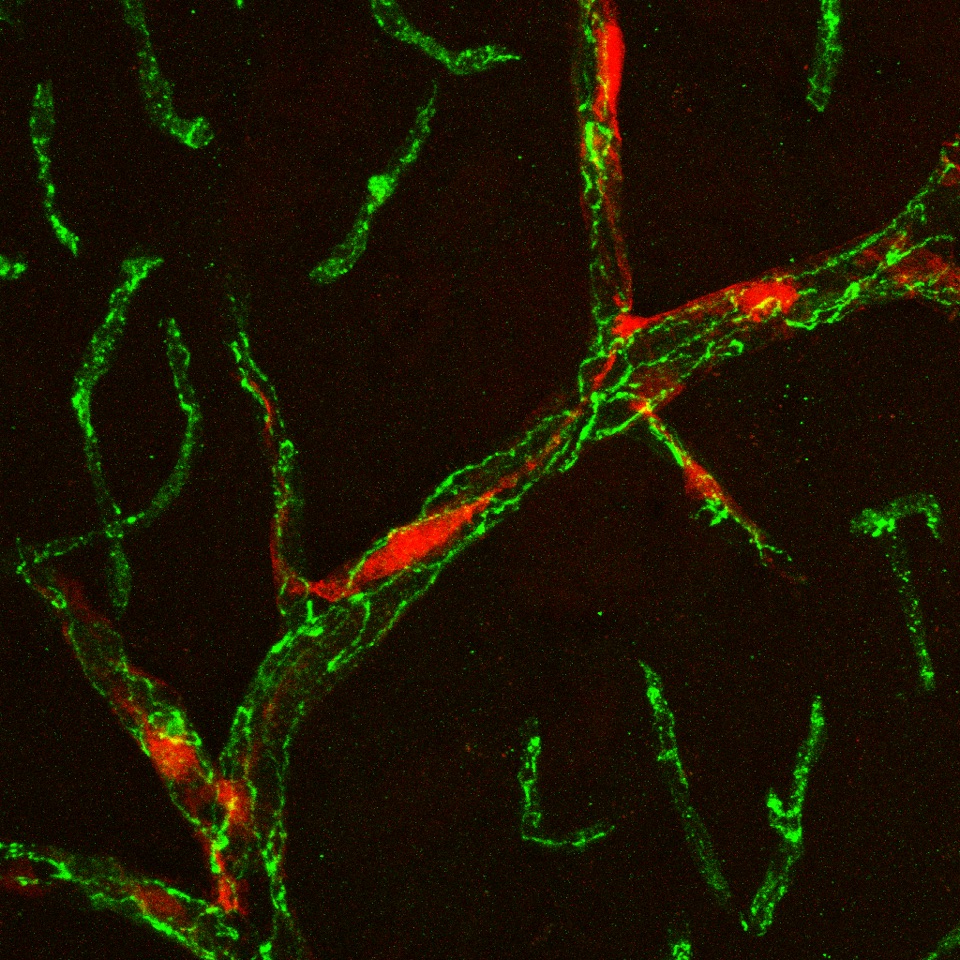
2. To test the hypothesis that albumin extravasation into the brain is reliant on monocyte migration
across the BBB. First, we will determine if microglial activation enhances monocyte and FITC-albumin translocation across an in vitro endothelial barrier. We will then determine if preventing monocyte migration across the barrier, via CCR2 antagonism or Ccr2 KO, decreases FITC-albumin translocation. Finally, the consequences of depleting brain-resident microglia in vivo prior to SE induction will be examined with the primary outcome measures being monocyte brain infiltration, albumin extravasation into the brain, neurodegeneration, and neuroinflammation in both the systemic pilocarpine and intra-amygdala kainate (IAK) models
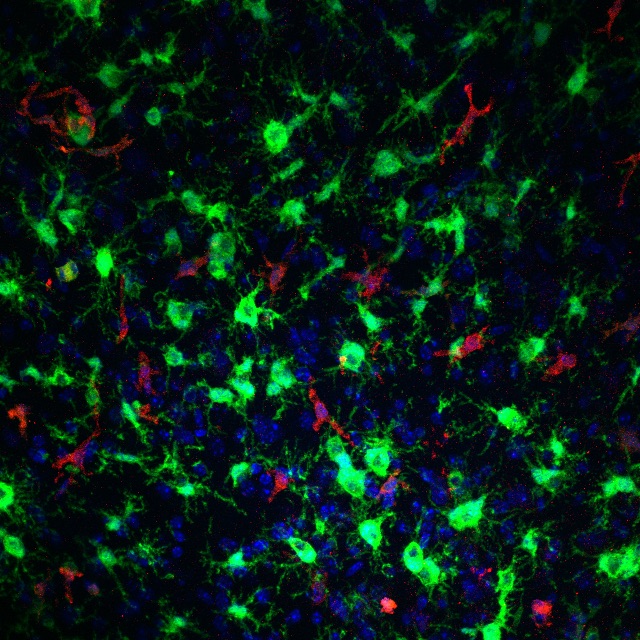
A second project in the laboratory asks if seizure-induced monocyte brain infiltration is a dominant driver of Alzheimer’s disease pathology, associated brain inflammation, and behavioral decline. If this project is successful we will have identified brain-invading monocytes as a key link between seizure activity and enhanced AD-related phenotypes. In addition to anti-seizure treatments, strategies aimed at preventing peripheral monocyte recruitment to the brain could be utilized to attenuate AD progression in those patients displaying epileptiform activity. This project currently funded through a three-year grant from the BrightFocus Foundation.
Varvel NH, Grathwohl SA, Degenhardt K, Resch C, Bosch A, Jucker M, Neher JJ. (2015) Replacement of brain-resident myeloid cells does not alter cerebral b-amyloid deposition. J Exp Med 212(11):1803-9 PMCID: PMC4612086
Varvel NH, Neher JJ, Bosch A, Wang W, Ransohoff RM, Miller RJ, Dingledine R. (2016) Infiltrating monocytes promote brain inflammation and exacerbate neuronal damage after status epilepticus. PNAS. 113(38):E5665-74.

