Research Interests of the Hepler Lab
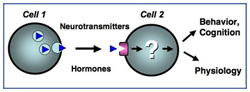
Cell-cell communication is essential for all aspects of cell and organ physiology. Cells communicate with one another by chemical messengers (e.g. hormones and neurotransmitters) that elicit their effects by recognizing and binding specific cell surface receptors to initiate cell signaling. The most prominent class of receptors is the family of G
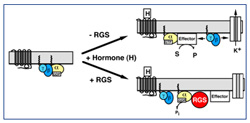
Recently, a class of signaling molecules known as the RGS proteins (regulators of
Genetic analysis of rare human variants in Regulators of G Protein Signaling (RGS) Proteins and their roles in physiology and disease
Montanez-Miranda C, Perszyk RE, Harbin NH, Okalova J, Ramineni, S, Traynelis SF, and Hepler JR (2022) Functional assessment of cancer-linked mutations in sensitive regions of RGS proteins predicted by 3DMTR analysis. Mol Pharmacol., in press. Friedman PA, Sneddon WB, Mamonova T, Montanez-Miranda C, Ramineni S, Harbin NH, Squires KE, Gefter JV, Magyar CE, Emlet DR, Hepler JR (2022) RGS14 regulates PTH- and FGF23-sensitive NPT2A-mediated renal phosphate uptake via binding to the NHERF1 scaffolding protein. J Biol Chem. May;298(5):101836. doi: 10.1016/j.jbc.2022.101836. Epub 2022 Mar 17. PMID: 35307350 Squires KE, Gerber KJ, Tillman MC, Lustberg DJ, Montañez-Miranda C, Zhao M, Ramineni S, Scharer CD, Saha RN, Shu FJ, Schroeder JP, Ortlund EA, Weinshenker D, Dudek SM, Hepler JR. (2020) Human genetic variants disrupt RGS14 nuclear shuttling and regulation of LTP in hippocampal neurons. J Biol Chem. 296:100024. doi: 10.1074/jbc.RA120.016009. Epub 2020 Nov 22. PMID: 33410399 Squires, K.H., Montanez-Miranda, C., Pandya, R. R., Torres, M.P. and Hepler, J.R. (2018) Genetic analysis of rare human variants in Regulators of G Protein Signaling (RGS) Proteins and their roles in physiology and disease. Pharmacological Reviews, Jul;70(3):446-474. Mar 30: Online at Pharmalogical Reviews.
RGS proteins as novel scaffolding proteins that integrate G protein pathways with other signaling pathways in neurons
Montanez-Miranda C, Bramlett SN, Hepler JR (2022) RGS14 expression in CA2 hippocampus, amygdala, and basal ganglia: Implications for human brain physiology and disease. Hippocampus, submitted. Harbin NH, Bramlett SN, Montanez-Miranda C, Terzioglu G, Hepler JR (2021) RGS14 Regulation of Post-Synaptic Signaling and Spine Plasticity in Brain. Int J Mol Sci. Jun 25;22(13):6823. doi: 10.3390/ijms22136823. PMID: 34201943 Foster SL, Lustberg DJ, Harbin NH, Bramlett SN, Hepler JR*, Weinshenker D.* (2021) RGS14 modulates locomotor behavior and ERK signaling induced by environmental novelty and cocaine within discrete limbic structures. Psychopharmacology (Berl). Oct;238(10):2755-2773. doi: 10.1007/s00213-021-05892-x. Epub 2021 Jun 29. PMID: 34184126 *Co-corresponding authors Gerber KJ, Dammer EB, Duong DM, Deng Q, Dudek SM, Seyfried NT, Hepler JR (2019) Specific Proteomes of Hippocampal Regions CA2 and CA1 Reveal Proteins Linked to the Unique Physiology of Area CA2. J Proteome Res. Jun 7;18(6):2571-2584. doi: 10.1021/acs.jproteome.9b00103. Epub 2019 May 14. PMID: 31059263 Abstract Link: https://pubmed.ncbi.nlm.nih.gov/31059263/ Gerber KJ, Squires KE, Hepler JR. (2018) 14-3-3γ binds regulator of G protein signaling 14 (RGS14) at distinct sites to inhibit the RGS14:Gαi-AlF4- signaling complex and RGS14 nuclear localization. J Biol Chem. Sep 21;293(38):14616-14631. doi: 10.1074/jbc.RA118.002816. Epub 2018 Aug 9. PMID: 30093406 Evans, P.R., Gerber, K.J., Ramineini, S., Yang. J., Griffin, P., Seyfried, N.J., and Hepler, J.R. (2018) Interactome Analysis Reveals Regulator of G Protein Signaling 14 (RGS14) is a Novel Calcium/ Calmodulin (Ca++/CaM) and CaM Kinase II (CaMKII) binding partner. J Proteome Res., Apr 6;17(4):1700-1711. doi: 10.1021/acs.jproteome.8b00027. Epub 2018 Mar 20. Online at BIORXIV as preprint: MS ID#: BIORXIV/2018/247270 Brown, N.E., Lambert, N.A and Hepler, J.R. (2016) RGS14 regulates the lifetime of Gα-GTP signaling but does not prolong Gβγ signaling following receptor activation in live cells. Pharmacol Res Perspectives. Aug 18;4(5):e00249 Brown, N.E., Goswami, D., Branch, M.R., Ramineni, S., Ortlund, E.A., Griffin, P.R. and Hepler, J.R. (2015) Integration of G protein alpha (Gα) Signaling by the Regulator of G protein Signaling 14 (RGS14). J. Biol. Chem., Apr 3;290(14):9037-49. Brown, N.E., Blumer, J.B., and Hepler, J.R. (2015) Bioluminescence Resonance Energy Transfer to Detect Protein-Protein Interactions in Live Cells. In: Protein-Protein Interactions: Methods and Applications, Methods in Molecular Biology, 1278:457-65. Vellano, C.P., Brown, N.E., Blumer, J.B. and Hepler, J.R. (2013) Assembly and function of the regulator of G protein signaling 14 (RGS14):H-Ras complex is regulated by Gαi1 and a Gi-linked GPCR. J. Biol. Chem., 288(5):3620-31. doi: 10.1074/jbc.M112.440057. Epub 2012 Dec 17. PMID: 23250758 Zhao, P., Nunn, C., Ramineni, S., Hepler, J.R. and Chidiac, P. (2013) The Ras-binding domain of RGS14 regulates its functional interactions with heterotrimeric G proteins. J. Cell. Biochem., 114(6): 1414-1423. doi: 10.1002/jcb.24483. Vellano, C.P., Maher, E.M., Hepler, J.R. and Blumer, J.B. (2011) G protein coupled receptors and Ric-8A both regulate the Regulator of G protein Signaling 14 (RGS14):Gαi1 complex in living cells. J. Biol. Chem. 286(44): 38659-38669 Vellano CP, Shu FJ, Ramineni S, Yates CK, Tall GG, Hepler, J.R. (2011) Activation of the regulator of G protein signaling 14-Gαi1-GDP signaling complex is regulated by resistance to inhibitors of cholinesterase-8A.Biochemistry 50(5):752-62 Shu, F.J., Ramineni, S and Hepler, J.R. (2010) RGS14 is a multifunctional scaffold that integrates G protein and H-Ras/Raf/MAP kinase signaling pathways. Cellular Signalling, 22(3):366-76. Hepler, J.R., Cladman, W. Ramenini, S. Hollinger, S. and Chidiac, P. (2005) Novel activity of RGS14 on Goα and Giα nucleotide binding and hydrolysis independent of its RGS domain and GDI activity. Biochemistry, 44, 5495-5502. Hollinger, S., Ramineni, S., and Hepler, J.R. (2003) Phosphorylation of RGS14 by protein kinase A modulates its activity toward Giα, Biochemistry, 42, 811-819. Hollinger, S., Taylor, J.B., Hoag-Goldman, E.M., and Hepler, J.R. (2001) RGS14 is a bifunctional regulator of Gai/o that exists in multiple populations in brain. J. Neurochem., 79, 941-949. Rose, J.J., Taylor, J.B., Shi, J., Jones, P.G, Cockett, M.R. and Hepler, J.R. (2000) RGS7 is palmitoylated and exists as biochemically distinct subpopulations in brain.
Shu F-J, Ramenini,S., Amyot, W.M., and Hepler, J.R. (2007) Selective interactions between Giα1 and Giα3 and the GoLoco/GPR domain of RGS14 influence its dynamic subcellular localization. Cellular Signalling, 17: 383-389.
J. Neurochem. 75, 2103-2112.
RGS protein regulation of hippocampal-based learning, memory and synaptic plasticity
Evans, P.R., Parra-Bueno, P., Smirnov, M.S., Lustberg, D.J., S., Dudek, S.M., Hepler, J.R.* and Yasuda, R.* (2018) RGS14 restricts plasticity in hippocampal CA2 by limiting postsynaptic calcium signaling. eNeuro, Jun 4;5(3). pii: ENEURO.0353-17.2018.. April 30, doi: 10.1523/ENEURO.0353-17.2018 Online at eNeuro * Co-corresponding authors
Squires*, K.E., Gerber*, K.J., Pare, J., Branch, M.R., Smith, Y., and Hepler, J.R. (2017) Regulator of G Protein Signaling 14 (RGS14) is Expressed Pre- and Postsynaptically in Neurons of Hippocampus, Basal Ganglia, and Amygdala of Monkey and Human Brain. Brain Structure and Function, doi: 10.1007/s00429-017-1487-y. [Epub ahead of print] PMID: 28776200 * Co-first authors
Branch, M.R. and Hepler, J.R. (2017) Endogenous RGS14 is a cytosolic-nuclear shuttling protein that localizes to juxtanuclear membranes and chromatin-rich regions of the nucleus.
PLOS ONE, 12(9):e0184497. doi: 10.1371/journal.pone.0184497
Evans, P.R., Dudek, S. M., and Hepler, J.R. (2015) Regulator of G Protein Signaling 14: A Molecular Break on Synaptic Plasticity Linked to Learning and Memory. Prog Mol Biol Transl Sci., in press.
Evans, P.R., Lee, S.E., Smith, Y., and Hepler, J.R. (2014) Postnatal Developmental Expression of Regulator of G Protein Signaling 14 (RGS14) in the Mouse Brain. J. Comp. Neurol, 522 (1): 186-203. (DOI:10.1002/cne.23395 (online pub, Nov 26, 2013).
Vellano, C.P, Lee, S.E., Dudek, S.M. and Hepler, J.R. (2011) RGS14 at the interface of hippocampal signaling and synaptic plasticity.Trends in Pharmacological Sciences32(11):666-674.
Lee, S.E., Simons, S.B., Heldt, S.A., Zhou, M., Schroeder, J.P, Cowan, D.P., Vellano, C.P., Feng, Y., Sweatt, J.D., Weinshenkar, D., Ressler, K.J., Dudek, S.M. and Hepler, J.R. (2010) RGS14 is a natural suppressor both of synaptic plasticity in CA2 neurons and hippocampal-based learning and memory. Proceedings of the National Academy of Sciences, USA, 107(39):16994-8
RGS14 is dubbed “The Homer Simpson Gene”
RGS14 as a multifunctional nuclear-cytoplasmic shuttling protein
Squires KE, Gerber KJ, Tillman MC, Lustberg DJ, Montañez-Miranda C, Zhao M, Ramineni S, Scharer CD, Saha RN, Shu FJ, Schroeder JP, Ortlund EA, Weinshenker D, Dudek SM, Hepler JR. (2020) Human genetic variants disrupt RGS14 nuclear shuttling and regulation of LTP in hippocampal neurons. J Biol Chem. 296:100024. doi: 10.1074/jbc.RA120.016009. Epub 2020 Nov 22. PMID: 33410399
Gerber KJ, Squires KE, Hepler JR. (2018) 14-3-3γ binds regulator of G protein signaling 14 (RGS14) at distinct sites to inhibit the RGS14:Gαi-AlF4- signaling complex and RGS14 nuclear localization. J Biol Chem. Sep 21;293(38):14616-14631. doi: 10.1074/jbc.RA118.002816. Epub 2018 Aug 9. PMID: 30093406
Branch, M.R. and Hepler, J.R. (2017) Endogenous RGS14 is a cytosolic-nuclear shuttling protein that localizes to juxtanuclear membranes and chromatin-rich regions of the nucleus. PLOS ONE, 12(9):e0184497. doi: 10.1371/journal.pone.0184497
Shu FJ, Ramineni S, Amyot W, Hepler JR. (2007) Selective interactions between Gi alpha1 and Gi alpha3 and the GoLoco/GPR domain of RGS14 influence its dynamic subcellular localization. Cell Signal. Jan;19(1):163-76. doi: 10.1016/j.cellsig.2006.06.002. Epub 2006 Jul 25. PMID: 16870394
Direct RGS protein regulation of GPCR/G protein signaling complexes
Brown, N.E., Lambert, N.A and Hepler, J.R. (2016) RGS14 regulates the lifetime of Gα-GTP signaling but does not prolong Gβγ signaling following receptor activation in live cells. Pharmacol. Res Perspectives. 18;4(5):e00249. Brown, N.E., Blumer, J.B., and Hepler, J.R. (2015) Bioluminescence Resonance Energy Transfer to Detect Protein-Protein Interactions in Live Cells. In: Protein-Protein Interactions: Methods and Applications, Methods in Molecular Biology, 1278:457-65. Ghil, SH, McCoy, K.L. and Hepler, J.R. (2014) RGS2 and RGS4 form distinct G protein-dependent complexes with protease activated receptor-1 (PAR1) in live cells. PLoS onE, 9(4):e95355. Doi:10.1371. Hepler, J.R. (2014) G protein coupled receptor signaling complexes in live cells. Cellular Logistics, 4(1):e29392 McCoy, K.L., and Hepler, J.R. (2009) RGS proteins as central components of the G protein coupled receptor signaling complex. Progress in Mol. Biol. Translational. Sci, 86; 49-74. Gu, S., He, J., Ho, W.T., Ramineni, S., Thal D.M., Natesh, R., Tesmer, J.J.G., Hepler, J.R. and Heximer, S.P. (2007). Unique hydrophobic extension of the RGS2 amphipathic helix domain imparts increased plasma membrane binding and function relative to other RGS R4/B subfamily members. J. Biol. Chem., 282: 33064-75. Neitzel, K.L. and Hepler, J.R. (2006) Cellular mechanisms that determine selective regulation of G protein coupled receptor signaling by RGS proteins. Seminars in Developmental and Cellular Biology, 17: 383-389. Roy, A.A., Baragli, A., Bernstein, L.S., Hepler, J.R., Hebert, T.E., and Chidiac, P. (2006) RGS2 interacts with adenylyl cyclase in living cells. Cellular Signalling., 18:336-48. Hague, C., Bernstein, L.S., Ramenini, S., Chen, Z.J., Minneman, K.P. and Hepler, J.R. (2005) Selective inhibition of α1A-adrenergic receptor signaling by RGS2 association with the receptor third intracellular loop. J .Biol Chem., 280, 27289-27295. Bernstein, L.S., Ramineni, S., Chris Hague, Wendy Cladman, Peter Chidiac, Levey, A.I. and Hepler, J.R. (2004) RGS2 binds directly and selectively to the M1 muscarinic cholinergic receptor third intracellular loop to modulate Gq/11α signaling. J. Biol. Chem., 279, 21248-21256. Hepler, J.R. (2003) RGS protein and G protein interactions: A little help from their friends.Molecular Pharmacology., 64, 547-549. Cunningham, M., Waldo, G.L., Hollinger, S., Hepler, J.R. and Harden, T.K. (2001) Protein kinase C phosphorylates RGS2 and modulates its capacity for negative regulation of G11α signaling. J. Biol. Chem., 276, 5438-5444. Heximer, S. P., Srinivasa, S.P., Bernstein, L.S., Bernard, J.L., Linder, M.E., Hepler, J.R. and Blumer, K.J. (1999) G protein selectivity is a determinant of RGS2 function. J. Biol. Chem., 274, 34253-34259. Saugstad, J.A., Marino, M.J., Folk, J.A., Hepler, J.R. and Conn, P.J. (1998) RGS4 inhibits signaling by group I metabotropic glutamate receptors. J. Neuroscience, 18: 905-913. Heximer, S.P., Watson, N., Linder, M.E., Blumer, K.J. and Hepler, J.R. (1997) RGS2/GOS8 is a selective inhibitor of Gqα function. Proc. Natl. Acad. Sci. USA, 94: 14389-14393.
Diversity of protease activated receptor (PAR) signaling in brain (in collaboration with the Traynelis lab at Emory)
Ghil, SH, McCoy, K.L. and Hepler, J.R. (2014) RGS2 and RGS4 form distinct G protein-dependent complexes with protease activated receptor-1 (PAR1) in live cells. PLoS ONE, 9(4):e95355. Doi:10.1371. McCoy, K.L., Gyoneva, S., Vellano, C.P., Smrcka A.V., Traynelis, S.F. and Hepler, J.R. (2012) Protease-Activated Receptor 1 (PAR1) coupling to Gq/11 but not to Gi/o or G12/13 is mediated by discrete amino acids within the receptor second intracellular loop. Cellular Signalling, 24(6):1351-60). McCoy, K.L., Traynelis, S.F. and Hepler, J.R. (2010) PAR1 and PAR2 couple to overlapping and distinct sets of G proteins and linked signaling pathways to regulate cell physiology.Molecular Pharmacology, 77(6):1005-15. Mannaioni, G., Goldshmidt, A., Hamill, C., Yuan, H., Pedone, K. H., Junge, C. E., Lee, C.J., Yepes, M., Hepler, J.R., and Traynelis, S.F. (2008) Plasmin potentiates synaptic NMDA receptor function in rat hippocampal neurons through activation of PAR1. J. Biol. Chem., 283(29): 20600-20611. Nicole, O., Sorensen, S.D., Sastre, A. Hepler, J.R., Brat, D., McKeon, R., and Traynelis, S.F. (2005) Activation of protease activated receptor-1 (PAR-1) contributes to glial scar formation after brain injury. J. Neuroscience, 25, 4319-4329. Junge, C.E., Lee, J.C, Hubbard, K.B., Zhang, Z., Olsen, J.J., Hepler, J.R., Brat, D.J., Traynelis, S.F. (2004) Protease activated receptor-1 (PAR-1) in human brain: localization and functional expression in astrocytes. Experimental Neurology, 188, 94-103. Sorensen, S.D., Nicole, O., Peavy, R.D., Montoya, L.M., Lee, J.C., Murphy, T.J., Traynelis, S.,F. and Hepler, J.R. (2003) Common signaling pathways link activation of murine PAR-1, LPA and S1P receptors to proliferation of astrocytes. Molecular Pharmacology., 64, 1199-1209.
Cell signaling diversity of Gqa family members
Pedone, K.H. and Hepler, J.R. (2007) The importance of amino terminal polycysteine and polybasic sequences in determining G14α and G16α palmitoylation, plasma membrane targeting and signaling function. J. Biol. Chem. 282(35): 25199-212.
Hubbard, K.B and Hepler, J.R. (2005) Cell signaling diversity of the Gqα family of heterotrimeric G proteins. Cellular Signalling, 18:135-50.
Peavy, R.B., Hubbard, K.B., Lau, A.G., Fields, B., Lee, T.T., Gernert, K., Murphy, T. J., and Hepler, J.R. (2005) Differential effects of Gqα, G14α and G15α on vascular smooth muscle cell survival and gene expression profiles. Molecular Pharmacology, 67, 2102-2114.

