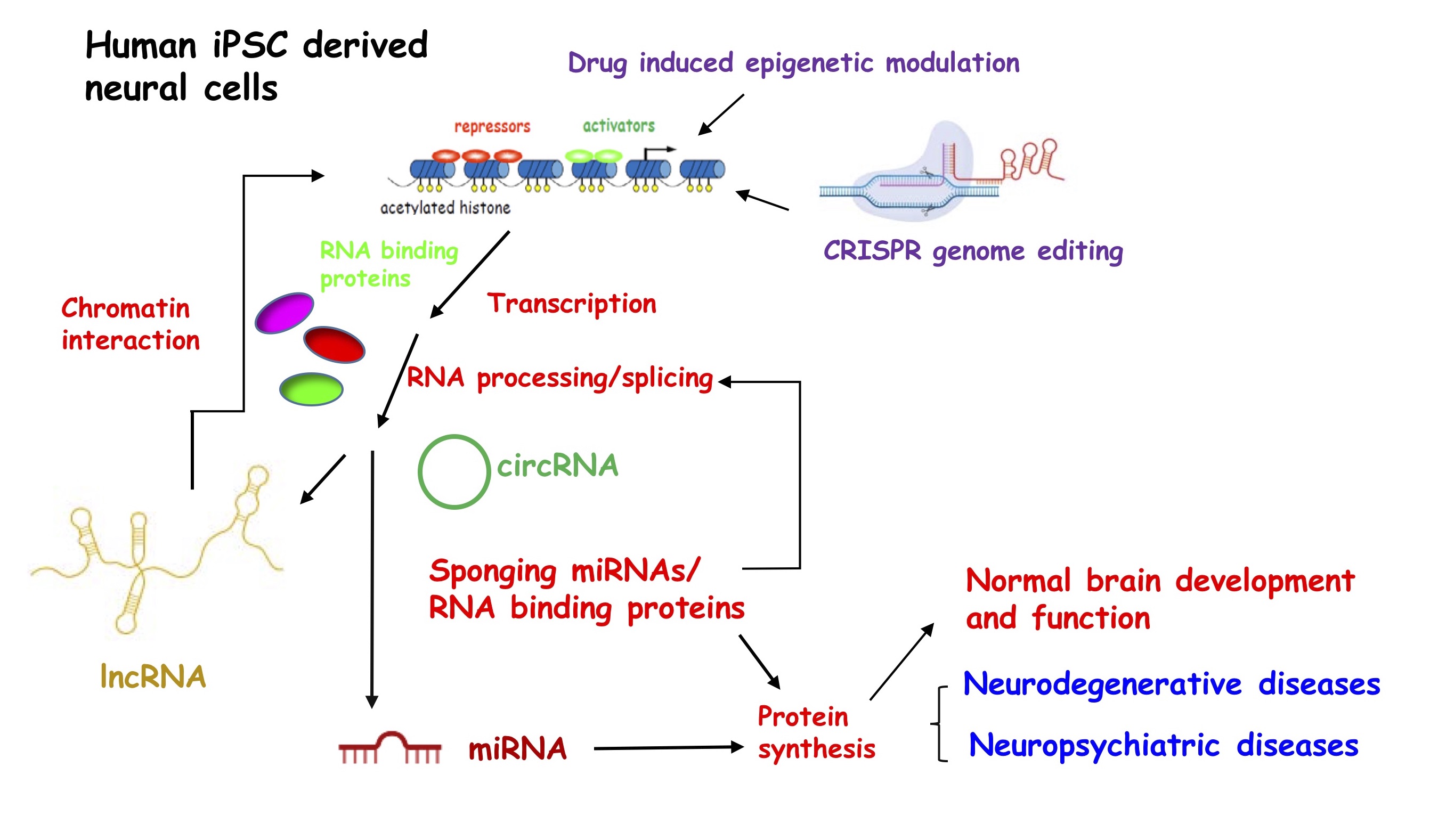
We are interested in how dysregulation of coding and non-coding RNAs lead to malfunction of neurons and glia in neuropsychiatric and neurological diseases. Taking a multidisciplinary strategy and a combination of molecular, cellular, genetics and pharmacological approaches, our current research focuses on the following directions:
Long non-coding RNAs in normal and diseased brains
Only ~one percent of the human genome encodes proteins while the rest produces the noncoding transcriptome. Unlike the highly conserved small non-coding RNAs (e.g. microRNAs), long noncoding RNAs (lncRNAs) contain greater than 200 nucleic acids in either linear or circular form, often are poorly conserved, enriched in the human brain but dysregulated in neuropsychiatric and neurological diseases. Thus, lncRNAs are thought to underline human brain sophistication and fragility, although their regulation and function remain vastly unknown.
We investigate molecular mechanisms that control the biogenesis of lncRNAs, circular RNAs and microRNAs, in human neuronal/glial lineages derived from induced pluripotent stem cells (iPSCs), focusing on risk factors for neuropsychiatric disease and neurological disorders. Moreover, we aim to delineate the functional integration of small and long non-coding RNAs, RNA binding proteins, and epigenetic chromatin modulation in normal brain development and how dysregulation leads to neural developmental abnormalities in cognitive brain disorders.
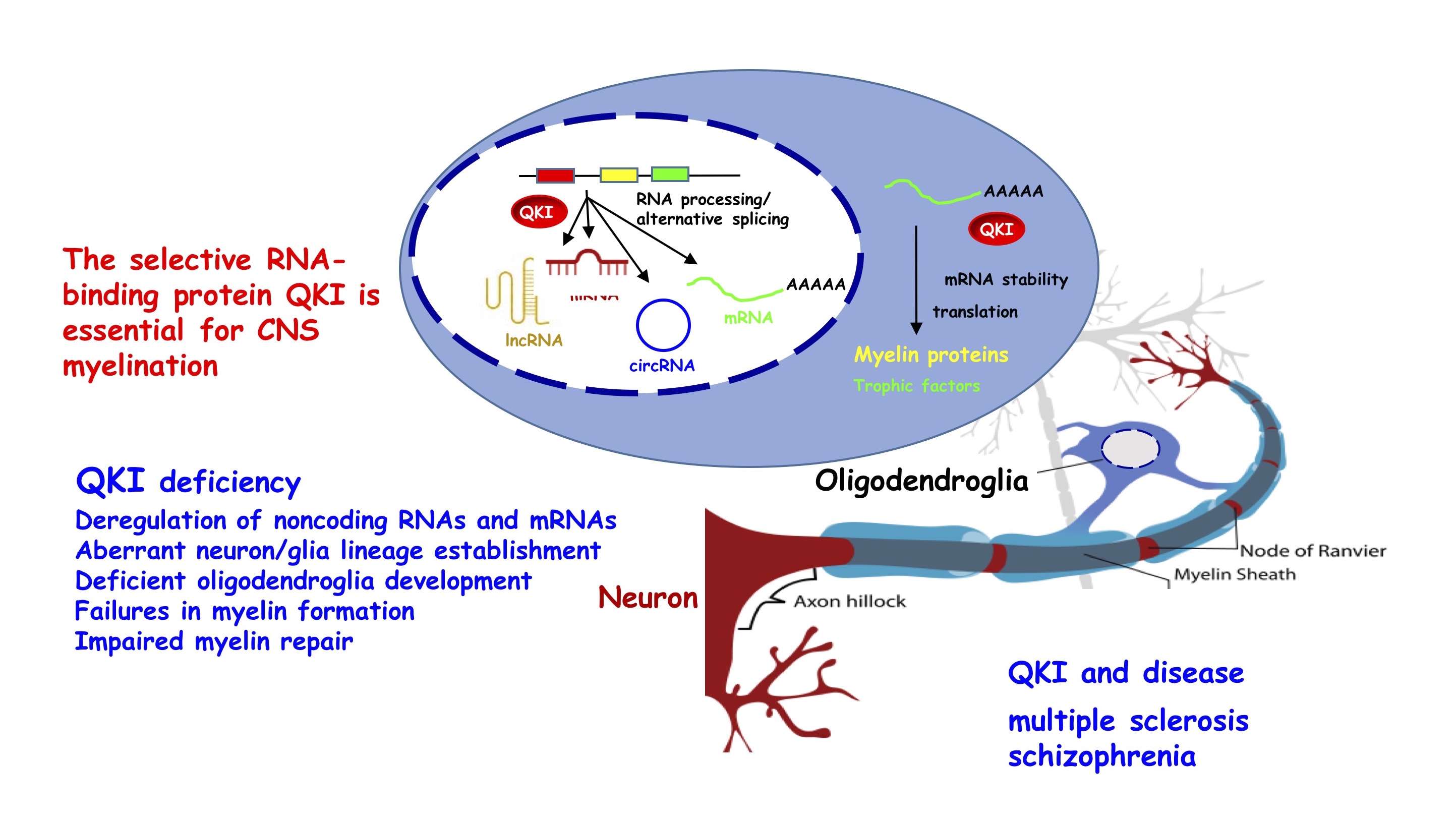
The QKI-pathway in myelin disorders
The loss of CNS myelin integrity causes numerous neurological diseases, represented by multiple sclerosis. In addition, myelin defects also contribute to the long range disconnectivity in schizophrenia. Our research focuses on the selective RNA-binding protein QKI, a key factor controlling CNS myelin formation and repair via regulating its coding and non-coding RNA targets. QKI is involved in multiple sclerosis and known as a risk factor of schizophrenia. We have identified complex regulation of mRNAs by QKI at the steps of mRNA splicing, stability and translation in response to developmental signaling. Our more recent efforts focus on QKI-dependent regulation of human non-coding RNAs, including microRNA and lncRNA processing, as well and circular RNA biogenesis and function. We have established human and rodent oligodendroglia cell cultures and mouse models to investigate QKI-dependent regulatory network. In addition, genetic and pharmacological approaches are employed to manipulate the QKI pathway in order to promote myelination.
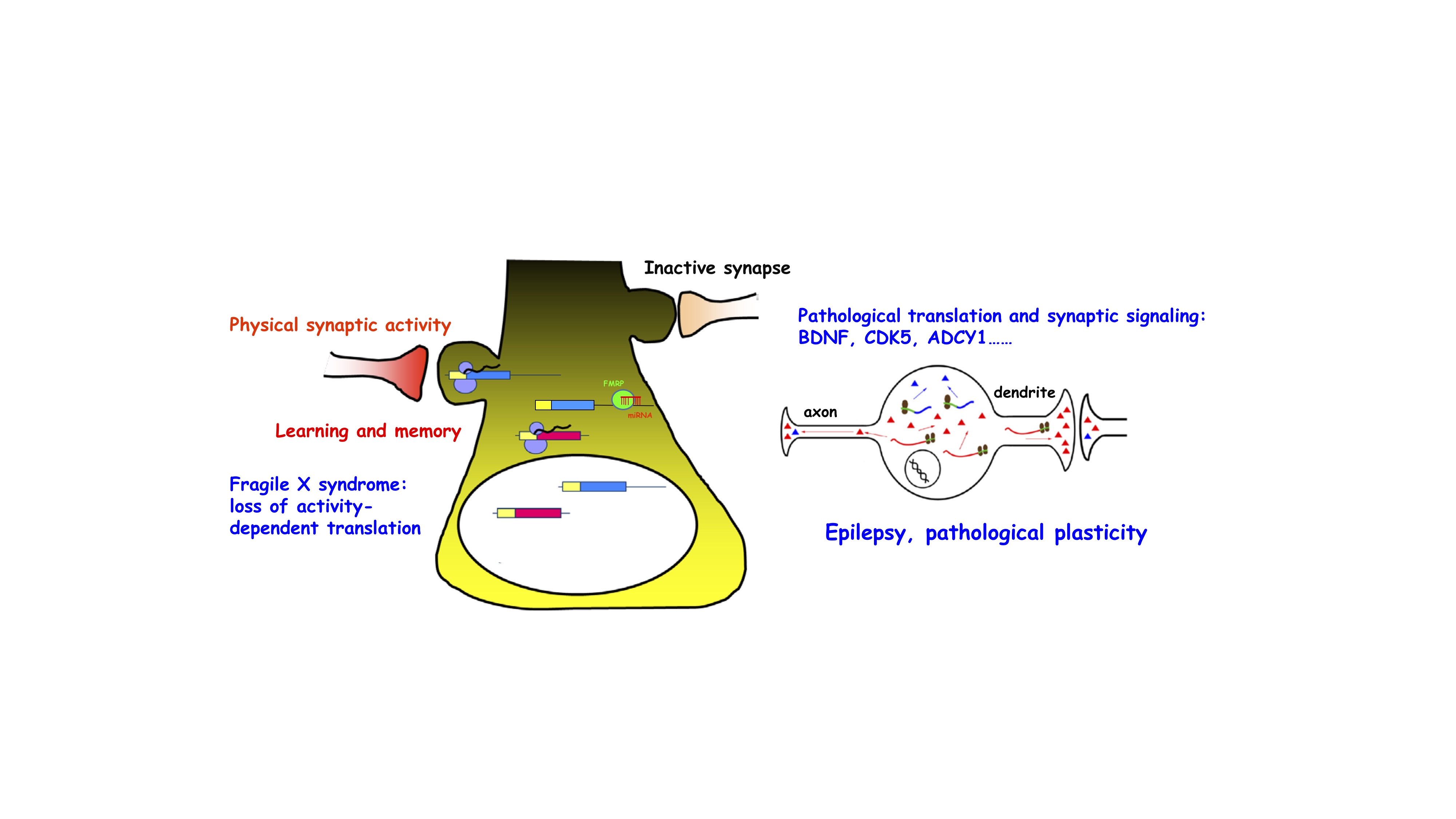
Translation regulation in neuronal network plasticity and brain disorders
Rapid and accurate regulation of protein synthesis in brain neurons, especially at the synapses, is a critical mechanism that governs neuronal network development, synaptic plasticity, and cognitive function. We investigate molecular mechanisms controlling mRNA translation and homeostasis of key signaling molecules that govern normal neuronal function but dysregulated in neurological and/or cognitive disorders. We focus on translation regulation that controls BDNF-TrkB signaling and Cdk5 signaling under physiological and pathological neuronal activity changes, including neuronal degeneration, stroke and epilepsy. In particular, we have a long standing interest in the fragile X mental retardation protein (FMRP), a selective RNA-binding protein that controls mRNA translation whose absence leads to fragile X syndrome, the leading cause of inherited intellectual disability. We investigate how FMRP collaborates with microRNAs to control translation of key synaptic signaling pathways in mouse models and human iPSC-derived 2D and 3D neuronal cultures, and testing whether pharmacological treatment can correct synaptic signals caused by FMRP deficiency.