Dr. Thomas Kukar
Dr. Thomas Kukar has a B.S. in Microbiology & Cell Science and a Ph.D. in Medicinal Chemistry, both from the University of Florida. His post-doctoral fellowship was at the Mayo Clinic in the Laboratory of Todd Golde, M.D., Ph.D. in the Department of Neuroscience. He later served as an Associate Consultant and Assistant Professor of Molecular Neuroscience in the College of Medicine at Mayo Clinic. Dr. Kukar joined the Department of Pharmacology in the School of Medicine at Emory University in 2010 and was promoted to Associate Professor in 2018. Dr. Kukar’s research goals are to understand the causes of age-associated neurodegenerative diseases with a long-term goal to develop treatments. His laboratory studies Alzheimer’s disease (AD), frontotemporal dementia (FTD), and amyotrophic lateral sclerosis (ALS).
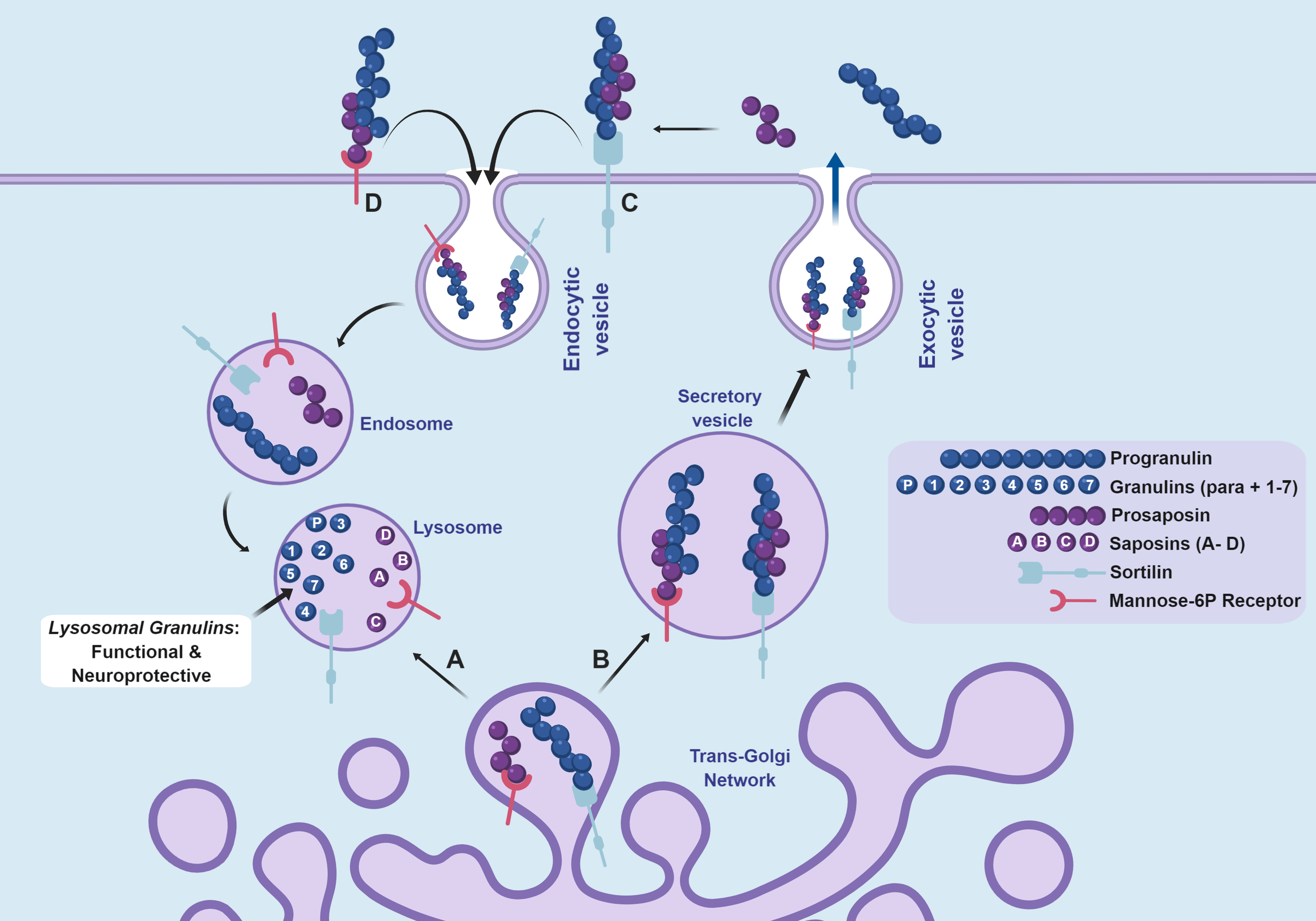
Recently, the Kukar lab has made exciting progress in their efforts to understand how mutations in the progranulin gene (GRN) increase the risk of AD or cause FTD. FTD is a fatal brain disease that is the most common cause of dementia in people under the age of 60. Mutations in GRN are a common cause of FTD and cause disease by lowering the circulating levels of the progranulin protein. However, the precise function of progranulin has been mysterious. Some evidence suggests progranulin functions as a growth factor or an anti-inflammatory protein. A recent study from the Kukar lab (Holler et al., eNeuro, 2017) reveals that progranulin also functions inside of cells, within the lysosome, where it is processed into smaller subunits called granulins. Although granulins are poorly understood, the dominant view was they function as proinflammatory molecules. In contrast, the Kukar lab discovery suggests that granulins are actually beneficial for lysosome health, not proinflammatory, and that loss of this function overtime leads to neurodegeneration. This discovery has led to funding for the Kukar lab’s research from the National Institutes of Health, the Bluefield Project to Cure FTD, the Alzheimer’s Association, and the Association for Frontotemporal Degeneration. This breakthrough finding has important implications for understanding the normal function of progranulin that will facilitate the development of drugs to treat FTD and AD, which are associated with decreased levels of progranulin and granulins.