
Dr. Yong Wan
Yong Wan, PhD, is a SOM Endowed Chair Professor at the School of Medicine and the Director of the Glenn Family Breast Center for Basic Research. The Wan Lab, among the first to utilize the state-of-the-art facilities at the Health Science Research Building (HSRB) II, is currently conducting cutting-edge patient-centered basic and translational studies for cancer therapy. Particularly, Wan Lab’s goal aims to define the molecular mechanisms of breast tumor initiation, progression, and metastasis, and to identify novel targets for therapeutic development. Wan Lab seeks to address how defects in protein posttranslational modifications (PTMs) would result in deregulated tumor immune checkpoint function, genomic instability, abnormal cell cycle, and aberrant signaling that predispose otherwise normal cells to become cancerous tumor cells or promote cancer progression and metastasis. The Wan Lab is conducting translational research in collaboration with medical oncologists while employing multidisciplinary approaches to advance drug development for cancer therapeutics, with a focus on PTMs.
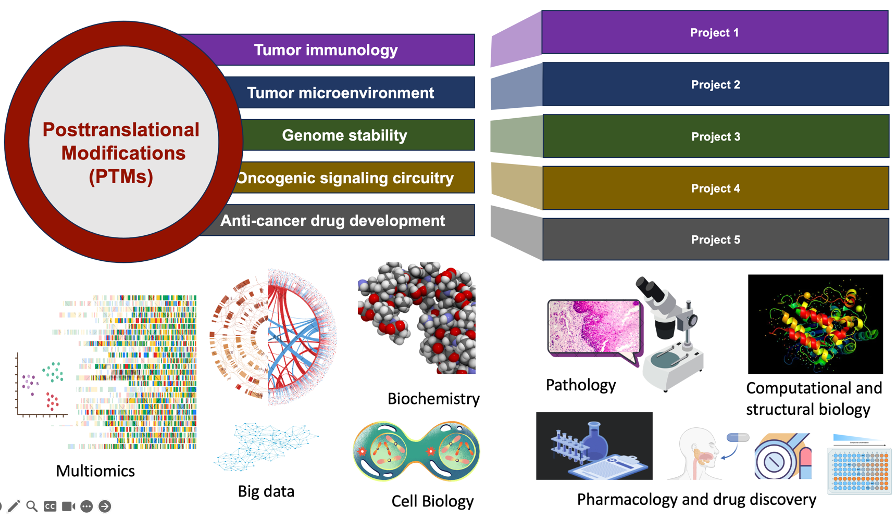
If genes represent the blueprint of life, then proteins as the functional units carry out biological processes. PTMs are biochemical decorations or alterations of proteins after they are synthesized, which not only profoundly impacts their structure and function but also increases the complexity and diversity to biological systems. Similar to how different combinations of condiments and spices can dramatically alter the flavor of a dish, these biochemical modifications affect a protein's stability, localization, interaction with other molecules, and activity. They play essential roles in nearly every biological process, including cell signaling, immune responses, cell death and differentiation, metabolism, and more. By attaching or detaching chemical groups, proteins can be switched on or off, guided to particular cellular destinations, or flagged for breakdown. Such precise control is not just crucial; it's the conductor orchestrating the symphony of cellular life, ensuring each note is played in perfect harmony for the entire organism to thrive. Malfunction of PTMs often leads to a variety of human diseases such as cancer. Understanding PTMs is not only fundamental to grasping how cells operate but also crucial for developing new therapies for diseases where these processes go awry.
Despite remarkable progress in breast cancer research in the past decades, especially in targeted therapy for Luminal A, Luminal B, and Her2 subtypes, patients with triple-negative breast cancer continue to face a dire lack of effective treatment options. Immunotherapy, which has shown great promise in treating various diseases, has also been severely restricted in breast cancer treatment due to its immune cold features, presenting an obstacle to its efficacy.
Aberrant PTMs play a role in diverse aspects of cancer biology, including tumor immune evasion, genome instability, tumorigenesis, and cancer drug resistance. Recent research by the Wan Lab has revealed a new mechanism governing the degradation of CD73, an immune checkpoint protein crucial for breast tumor immune evasion. These findings suggest the Yin-Yang regulation of CD73 by ubiquitylation and deubiquitylation, which orchestrates tumor immunosuppression and can be targeted for therapeutic approaches. The lab is also actively involved in multiple projects examining the impact of PTMs on the communication between tumors, immune cells, and adipocytes. Alongside their work on PRMT5, CDC20 protac, OTUD4-CD73, and MGAT1, they are actively identifying new therapeutic targets and agents.
"HSRB II’s dynamic research platform offers a multitude of interdisciplinary avenues and opportunities. As we walk down this corridor of discovery, we are constantly inspired by our colleagues' work with the most exciting and cutting-edge research tools such as machine learning, 3D bioprinting, advanced imaging, and many more," says Wan. “We look forward to collaborating with fellow scientists and clinicians to dive deeper into the mysteries of cancer and develop novel therapeutics that could benefit patients around the world.” Looking ahead, Wan and his team are focused on expanding Emory's breast cancer research, pioneering innovative anti-cancer therapies, and potentially establishing a dedicated center for PTM research. This effort aims to foster dynamic interdisciplinary collaboration by bringing together experts from pharmacology, oncobiology, drug discovery, and cancer care.

