Areas of Clinical Research Focus
- Acne
- Alopecia
- Atopic Dermatitis
- Bullous Diseases (Bullous Pemphigoid, Pemphigus Vulgaris)
- Cutaneous Oncology
- Hidradenitis suppurativa
- Prurigo nodularis
- Pruritus
- Psoriasis
- Vitiligo
- Urticaria
Enrolling Dermatology Trials
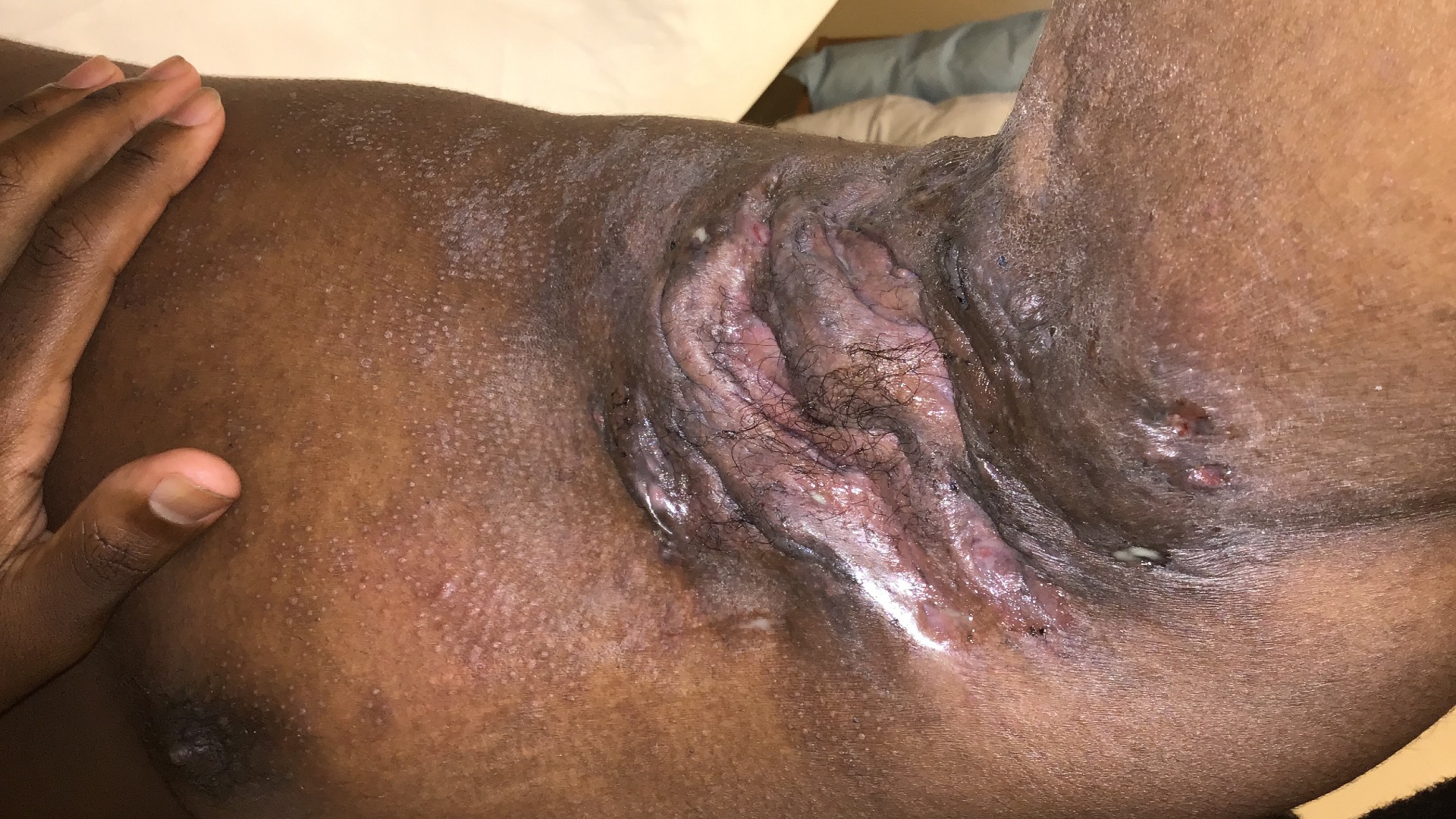
Pain in Hidradenitis Suppurativa (HS): Outcome Metrics and Improvement in Quality of Life
We are currently seeking participants for a research study about pain in hidradenitis suppurativa (HS).
This 16-week study will include 2 in-person visits with additional remote visits. The study team will ask you to do the following:
- To have your skin examined
- To fill out questionnaires that ask about your quality of life and your pain
- To have some photos taken of your skin lesions
- To have a blood sample drawn
- To undergo quantitative sensory testing (QST) on the back of your hand and on select HS lesions
Inclusion criteria
- Dermatologist-confirmed diagnosis of HS
- Ongoing HS activity
- Age ≥16 years – 80 years
- Ability to understand and read English in order to read questionnaires
- Reliable internet and telephone access
- Stated willingness to stay on the same HS disease-modifying therapy over the course of the study
Exclusion criteria
- Medical conditions other than HS causing chronic pain
- Medical conditions causing neuropathy such as diabetic neuropathies
- Change in systemic HS disease-modifying therapy in the 4 weeks prior to inclusion (for non-biologic), or 8 weeks prior (for biologics)
- New prescription of a sedative, NMDA receptor agonist, eugeroic antidepressant, or scheduled opioid within 4 weeks prior to inclusion
For more information on this study, please contact Kimwanna Norwood.
Upcoming Clinical Trials
A Phase 3, Placebo-controlled, Double-blind Study Assessing Rocatinlimab in Prurigo Nodularis
The main objective of this study will be to evaluate the efficacy of rocatinlimab compared with placebo at week 24 on the patient-reported outcome (PRO) measure of pruritus and overall clinical assessment score.
Inclusion Criteria:
- Age ≥ 18 years -100 years
- Clinical diagnosis of Prurigo Nodularis that has been present for at least 3 months.
- Has ≥ 20 prurigo nodularis nodules in total with bilateral distribution on both legs and/or arms and/or trunk.
Exclusion Criteria:
- Prurigo nodularis secondary to medications.
- Prurigo nodularis secondary to neurologic or psychiatric medical conditions.
- Treatment with any systemic biologic immunosuppressive or systemic biologic immunomodulatory therapy for prurigo nodularis or any other autoimmune, inflammatory, or allergic disease within 12 weeks or 5 half-lives, whichever is longer, prior to day 1 prerandomization.
For more information on this study, please contact Julie Bonds or visit ClinicalTrials.gov